
(1)��ѧƽ�ⳣ��K��ʾ���淴Ӧ�Ľ��г̶ȣ�KֵԽ��ʾ���淴Ӧ���е�Խ��ȫ��Kֵ��С���¶ȵĹ�ϵ�ǣ��¶����ߣ�Kֵ (�һ��������һ����С����������Ҳ���ܼ�С��)��
(2)��һ���Ϊ10 L�������У�ͨ��һ������CO��H2O����800��ʱ�������·�Ӧ��CO(g)+H2O(g) CO2(g)+H2(g)��H��0��CO��H2O�����ʵ���Ũ�ȱ仯��ͼ��ʾ����
CO2(g)+H2(g)��H��0��CO��H2O�����ʵ���Ũ�ȱ仯��ͼ��ʾ����
��0��4 minʱ���ƽ����Ӧ����v(CO)�� mol��L-1��min-1��
����800��ʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��K�� (Ҫ��д������ʽ����ֵ)��CO��ת���ʣ� ��
����800��ʱ������Ӧ��ʼʱ��������CO��H2O��Ũ�ȷֱ�Ϊ0.20 mol��L-1��0.80 mol��L-1����ﵽƽ��ʱCOת��ΪCO2��ת������ ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ijһ�ݻ�Ϊ5 L���ܱ������ڣ�����0.2 mol��CO��0.2 mol��H2O(g)���ڴ������ڵ������¸��¼��ȣ��������·�Ӧ��CO(g)��H2O(g)  CO2(g)��H2(g)����Ӧ�ų���������Ӧ��CO2��Ũ����ʱ��仯�������ͼ��ʾ��
CO2(g)��H2(g)����Ӧ�ų���������Ӧ��CO2��Ũ����ʱ��仯�������ͼ��ʾ��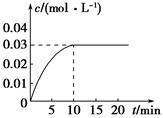
(1)����ͼ�����ݣ��ӷ�Ӧ��ʼ���ﵽƽ��ʱ��CO�Ļ�ѧ��Ӧ����Ϊ________����Ӧƽ��ʱc(H2)��________��
(2)�жϸ÷�Ӧ�ﵽƽ���������________(�����)��
��CO��С�Ļ�ѧ��Ӧ���ʺ�CO2��С�Ļ�ѧ��Ӧ�������
��CO��H2O��CO2��H2��Ũ�ȶ����
��CO��H2O��CO2��H2��Ũ�ȶ����ٷ����仯
�������淴Ӧ���ʶ�Ϊ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��5 L�ܱ������м���2 mol Fe(s)��1 mol H2O(g)��t1��ʱ��H2�����ʵ���Ϊ0.20 mol������t2��ʱǡ�ôﵽƽ�⣬��ʱH2�����ʵ���Ϊ0.35 mol��
(1)t1��t2���ʱ���ڵĻ�ѧ��Ӧ����v(H2)= ��
(2)����������2 mol Fe(s)����ƽ���ƶ� (�������Ӧ���������淴Ӧ������)������ͨ��1 mol H2O(g)�ٴδﵽƽ���H2���ʵ���Ϊ __________mol��
(3)�÷�Ӧ���淴Ӧ������ʱ��仯�Ĺ�ϵ��ͼ��t1ʱ�ı���ij���������ı�������� _________��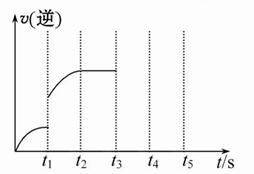
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��-��ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ��
��.SO2+2H2O+I2=H2SO4+2HI
��.2HI H2��+I2
H2��+I2
��.2H2SO4=2SO2+O2��+2H2O
(1)����������Ӧ�������ж���ȷ���� ��
a.��Ӧ�����ڳ����½���
b.��Ӧ����SO2�����Ա�HIǿ
c.ѭ���������貹��H2O
d.ѭ�������в���1 mol O2��ͬʱ����1 mol H2
(2)һ���¶��£���1 L�ܱ������м���1 mol HI(g)��������Ӧ��H2�����ʵ�����ʱ��ı仯��ͼ��ʾ��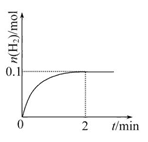
��0��2 min�ڵ�ƽ����Ӧ����v(HI)= ��
����ͬ�¶��£�����ʼ����HI(g)�����ʵ�����ԭ����2������ ��ԭ����2����
a.HI��ƽ��Ũ��
b.�ﵽƽ���ʱ��
c.ƽ��ʱH2���������
(3)ʵ������Zn��ϡ������ȡH2���������������й����Լ��е� ������H2�����ʽ�����
a.NaNO3 b.CuSO4 c.Na2SO4 d.NaHSO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)(�㽭�߿�)�������෴Ӧ����ij���(B)��ƽ��ѹǿ(pB)�������ʵ���Ũ��(cB)Ҳ�ɱ�ʾƽ�ⳣ��(����Kp)����ӦCH4(g)��H2O(g) CO(g)��3H2(g)����H��206.2 kJ��mol��1��Kp��________�������¶ȵ����ߣ���ƽ�ⳣ��________(�������С�����䡱)��
CO(g)��3H2(g)����H��206.2 kJ��mol��1��Kp��________�������¶ȵ����ߣ���ƽ�ⳣ��________(�������С�����䡱)��
(2)(���߿�)д��WO3(s)��3H2(g) W(s)��3H2O(g)�Ļ�ѧƽ�ⳣ������ʽΪ________��
W(s)��3H2O(g)�Ļ�ѧƽ�ⳣ������ʽΪ________��
(3)(�����߿�)�ں��ݾ���(������罻������)�����½���2A(g)��B(g)??2C(g)��D(s)��Ӧ�����±�����Ͷ�ϣ���Ӧ�ﵽƽ��״̬�������ϵѹǿ���ߡ������÷�Ӧ��ƽ�ⳣ�����¶ȵı仯��ϵ��________��
| ���� | A | B | C | D |
| ��ʼͶ��/mol | 2 | 1 | 2 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ�ݻ�������ܱ������г���һ����A��B��������Ӧ��xA(g)��2B(s) yC(g)����H��0����һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺
yC(g)����H��0����һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺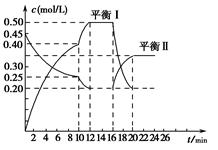
(1)��A��Ũ�ȱ仯��ʾ�÷�Ӧ0��10 min�ڵ�ƽ����Ӧ����v(A)��________��
(2)����ͼʾ��ȷ��x��y��________��
(3)0��10 min������ѹǿ________(���������䡱��С��)��
(4)�Ʋ��10 min�������߱仯�ķ�Ӧ����������________����16 min�������߱仯�ķ�Ӧ����������________��
�ټ�ѹ��������A��Ũ�ȡ�������C������������ �ݽ��¡��Ӵ���
(5)��ƽ����ƽ�ⳣ��ΪK1��ƽ����ƽ�ⳣ��ΪK2����K1________K2(�����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
һ�������������Ժ�CO2������Ӧ��Fe(s)��CO2(g) FeO(s)��CO(g)��H��0��1100��ʱ����ij�ܱ������м����������۲�����һ������CO2���壬��Ӧ������CO2�����CO�����Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
FeO(s)��CO(g)��H��0��1100��ʱ����ij�ܱ������м����������۲�����һ������CO2���壬��Ӧ������CO2�����CO�����Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��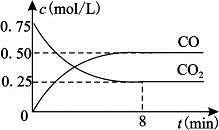
��1�����д�ʩ����ʹƽ��ʱK�������___________������ţ���
A�������¶� B������ѹǿC������һ����COD�������¶�
��2��8�����ڣ�CO��ƽ����Ӧ����v��CO��=___________mol/(L��min)��
��3��1100��ʱ��2L���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й���������
| ���� | �� | �� |
| ��Ӧ��Ͷ���� | 3molFe��2molCO2 | 4molFeO��3molCO |
| CO��Ũ��(mol/L) | C1 | C2 |
| CO2��������� | 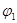 | 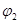 |
| ��ϵѹǿ��Pa�� | P1 | P2 |
| ��̬��Ӧ���ת���� | 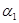 | 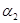 |
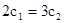 B��
B��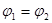 C��P1��P2D��
C��P1��P2D��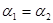
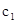 ��___________��
��___________��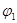 ��___________��
��___________��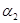 ��___________��
��___________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ȤС��̽����â��Na2SO4��10H2O��CaOΪԭ���Ʊ�Na2CO3��
��1����CaOˮ������â���γ�Na2SO4��Ca(OH)2��H2O��Ԫ��ϵ����Ӧ����ˣ�����Һ��ͨ��CO2�������õ�Na2CO3����Ԫ��ϵ�з�Ӧ�����ӷ���ʽΪ�� SO42��+ Ca(OH)2(s)+2H2O CaSO4��2H2O(s)+2 OH��
CaSO4��2H2O(s)+2 OH��
�÷�Ӧ��ƽ�ⳣ������ʽK=_________________________��
��Na2SO4��Ca(OH)2��H2O��Ԫ��ϵ������������ij���������ʣ�����pH="12.3" [��c(OH��)=0.02mol/L]����ʹ��Ӧ�ڳ����������С���Ӧ����ˣ�������Һ��ͨ��CO2����һ�������õ�Na2CO3��
��2����Na2SO4��Ca(OH)2��H2O��Ԫ��ϵ�в�ֱ��ͨ��CO2����������_______________________________________________________________��
��3�����ӵ����������������������д�����㣩��_____________��______________��
��4����ƽ���ƶ�ԭ�����������������ʵ����ɣ�____________________________________����HA��ʾ�����ӵ����ʣ����ܷ�Ӧ�����ӷ���ʽ��дΪ_______________________��
��5��Na2CO3��Һ�д���ˮ��ƽ�⣺CO32����H2O HCO3����OH��������˵���������_________��
HCO3����OH��������˵���������_________��
a����ˮϡ�ͣ���Һ���������ӵ�Ũ�ȶ���С
b��ͨ��CO2����ҺpH��С
c������NaOH���壬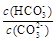 ��С
��С
d��ϡ����Һ��ƽ�ⳣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��4�֣���һ���Ϊ10L�ܱյ������У�ͨ��һ������CO��H2O��g������850��ʱ�������·�Ӧ��CO(g)+H2O(g)  CO2(g)+H2(g) ��H��0
CO2(g)+H2(g) ��H��0
��1��CO��H2OŨ�ȱ仯��ͼ����0��4 min��ƽ����Ӧ���ʦ�(CO)��_______ mol/��L��min������ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ �� 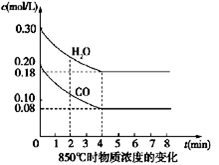
��2����������Щ���������ٷ����仯ʱ������������Ӧ�Ѵﵽƽ��״̬���� ��
| A����������ѹǿ |
| B�����������ܶ� |
| C��CO�����ʵ���Ũ�� |
| D���ܱ������зų����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com