��ͬѧ�����ͼ����ʾװ����֤ͭ��ϡ����ķ�Ӧ��������ˮ���ռ�NO����
��1��д���Թ�����Һ�з�����Ӧ�Ļ�ѧ����ʽ
3Cu+8HNO3=3Cu��NO3 �� 2+2NO��+4H2O
3Cu+8HNO3=3Cu��NO3 �� 2+2NO��+4H2O
��2����ʵ������й۲쵽�Թ��ڵ���Һ��
��
��
ɫ���ռ�������Թ��ڵ������
��
��
ɫ
��3����ͬѧ��Ϊ����Ȼ�ռ�����������һ����������������˵����Ӧ��һ������һ������������������
��ͭ��ϡ���ᷴӦ�������ϲ������˺���ɫ����
��ͭ��ϡ���ᷴӦ�������ϲ������˺���ɫ����
��ͭ��ϡ���ᷴӦ�������ϲ������˺���ɫ����
��ͭ��ϡ���ᷴӦ�������ϲ������˺���ɫ����
�йػ�ѧ����ʽΪ��
2NO+O2=2NO2
2NO+O2=2NO2
��4����ͬѧ�����ͼ����ʾװ�ý���ʵ�飬֤����ͭ��ϡ���ᷴӦ����һ����������ͬѧ�IJ������±���ʾ����ش�ʵ���е��й����⣮
| ʵ�鲽�� |
���� |
| 1��U����˼���ϡ����ֱ������U���Ҷ� |
��/ |
| 2�ø���ͭ˿�Ľ�����סU���Ҷˣ��۲����� |
������ ����ɫ����������ұ���Һ�����ɫ ����ɫ����������ұ���Һ�����ɫ |
| 3����Ӧֹͣ��������۲�ʵ������ |
������ ��ɫ����������Ӵ���������ɺ���ɫ ��ɫ����������Ӵ���������ɺ���ɫ |
��5���ӻ����ĽǶȿ�����ͬѧ��ʵ����ڵ�ȱ����
�������������������л���Ⱦ����
�������������������л���Ⱦ����
��
��6����ͬѧ��ͼ����ʾװ�ô���ͼ����U���Ҷ˵ĸ���ͭ˿�Ľ�������ʵ�飬����Ӧֹͣ��һ���������������Һ©���У��۲쵽��ʵ���������ͬѧʵ��ĵ�
3
3
�������ͬ����ͬѧҪ��һ���������������Һ©����Ӧ���е���ȷ�����Ǣ�
��Һ©���ϲ�������
��Һ©���ϲ�������
��
������Һ©���Ļ���
������Һ©���Ļ���
��
��Һ���뽺���Ӵ�ʱ�����رջ���
��Һ���뽺���Ӵ�ʱ�����رջ���
��7��ʵ�������ͬѧ���Һ©����ע������ˮ���������д�������Ļ�ѧ��Ӧ����ʽ
3NO2+H2O=2 HNO3+NO
3NO2+H2O=2 HNO3+NO
��8������32.64gͭ��140mLһ��Ũ�ȵ����ᷴӦ��ͭ��ȫ�ܽ⣬������NO��NO
2��������ڱ�״���µ����Ϊ11.2L��������NO
2�ķ�Ӧ�����Իش�
����ͭ�����պ÷�Ӧ��ȫ����ԭ������Һ�����ʵ���Ũ��Ϊ
10.86mol�qL-1
10.86mol�qL-1
�ڽ�����������ȫ���ͷź�����Һ�м���VmL��amol?L
-1��NaOH��Һ��ǡ��ʹ��Һ�е�Cu
2+ȫ��ת��Ϊ��������ԭ������Һ�����ʵ���Ũ��Ϊ
����֪NO��NO
2�Ļ����������������Ϻ��ܱ�ˮ��ȫ���ճ����ᣬ��Ҫʹ����11.2L������ȫ��ˮ���ճ����ᣬ�����������ı�״���µ�����
5.71
5.71
������������λС������������̣�
�����ɵ�һ������Ϊxmol����������Ϊymol��
3Cu+8HNO
3=3Cu��NO
3 ��
2+2NO��+4H
2O
3 2
1.5xmol xmol
Cu+4HNO
3��Ũ��=Cu��NO
3 ��
2+2NO
2��+2H
2O
1 2
ymol��ymol��
���Է�����Ϊ
����
��һ������Ϊ0.26mol����������Ϊ0.24mol��
4NO+2H
2O+3O
2=4HNO
3
4 3
0.26mol 0.195mol
4 NO
2+2H
2O+O
2=4HNO
341
0.24mol 0.06mol
������Ҫ����0.195mol+0.06mol=0.255mol�����Ϊ0.255mol��22.4L/mol=5.71L
�����ɵ�һ������Ϊxmol����������Ϊymol��
3Cu+8HNO
3=3Cu��NO
3 ��
2+2NO��+4H
2O
3 2
1.5xmol xmol
Cu+4HNO
3��Ũ��=Cu��NO
3 ��
2+2NO
2��+2H
2O
1 2
ymol��ymol��
���Է�����Ϊ
����
��һ������Ϊ0.26mol����������Ϊ0.24mol��
4NO+2H
2O+3O
2=4HNO
3
4 3
0.26mol 0.195mol
4 NO
2+2H
2O+O
2=4HNO
341
0.24mol 0.06mol
������Ҫ����0.195mol+0.06mol=0.255mol�����Ϊ0.255mol��22.4L/mol=5.71L
��



 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
 �������������������������������ռ�����
�������������������������������ռ�����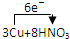 =3Cu��NO3��2+2NO��+4H2O
=3Cu��NO3��2+2NO��+4H2O =3Cu��NO3��2+2NO��+4H2O
=3Cu��NO3��2+2NO��+4H2O
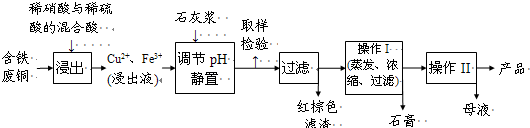
 ����ͭ��һ��Ӧ�ü���㷺�Ļ���ԭ�ϣ�ͭ������ϡ����ֱ�ӷ�Ӧ����ʵ���н�����Ũ����ֶ�μ��뵽ͭ����ϡ����Ļ�����У�����ʹ֮��Ӧ��ȫ��ͨ���������ᾧ�õ�����ͭ���壨װ����ͼ��ʾ����
����ͭ��һ��Ӧ�ü���㷺�Ļ���ԭ�ϣ�ͭ������ϡ����ֱ�ӷ�Ӧ����ʵ���н�����Ũ����ֶ�μ��뵽ͭ����ϡ����Ļ�����У�����ʹ֮��Ӧ��ȫ��ͨ���������ᾧ�õ�����ͭ���壨װ����ͼ��ʾ����