
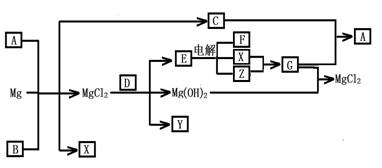
��12�֣�Mg���仯������Է�������ת�������ַ�Ӧ��������ˮ����ȥ������֪X��Y��ZΪ��̬���ʣ�B������ΪҺ̬��������D����ɫ��ӦΪ��ɫ��C��G���ð���̲�����A�����E��ˮ��Һ������ij�ֹ�ҵ������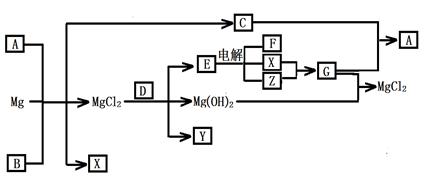
��1��д���������ʵĻ�ѧʽ
A Y
��2��д��C�ĵ���ʽ
��3��д��MgCl2��D��Ӧ�Ļ�ѧ����ʽ
��4������ˮ��ƽ�����۽���A+B+Mg��C+X+ MgCl2��ԭ��_______________________��
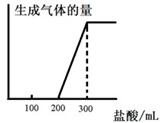
��5����0.1molCO2ͨ��1L����ΪF����Һ�У���ַ�Ӧ��������Һ����εμ����ᣬ�����������������ɵ������ ��ϵ����ͼ��ʾ����ԭF��Һ��Ũ��Ϊ mol/L��
��ϵ����ͼ��ʾ����ԭF��Һ��Ũ��Ϊ mol/L��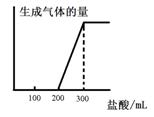
 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
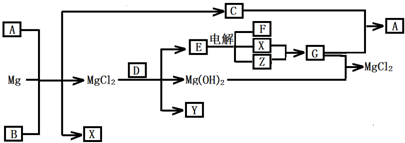
(14��)Mg���仯������Է�������ת�������ַ�Ӧ��������ˮ����ȥ������֪X��Y��ZΪ��̬���ʣ�B������ΪҺ̬��������D����ɫ��ӦΪ��ɫ��C��G���ð���̲�����A�����E��ˮ��Һ������ij�ֹ�ҵ������
��1��д���������ʵĻ�ѧʽ
A Y
��2��д��C�ĵ���ʽ
��3��д��A��Һ�и�����Ũ�ȵĴ�С˳���ɴ�С��
��4��д��MgCl2��D��Ӧ�Ļ�ѧ����ʽ
��5��д�����E��Һ�����ӷ���ʽ
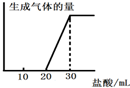
��6����0.1molCO2ͨ��1L����ΪF����Һ�У���ַ�Ӧ��������Һ����εμ����ᣬ�����������������ɵ�����Ĺ�ϵ��ͼ��ʾ����ԭF��Һ��Ũ��Ϊ mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ�������ѧ�߶���ѧ�ڵ�һ�ο��Ի�ѧ�Ծ� ���ͣ������
(14��)Mg���仯������Է�������ת�������ַ�Ӧ��������ˮ����ȥ������֪X��Y��ZΪ��̬���ʣ�B������ΪҺ̬��������D����ɫ��ӦΪ��ɫ��C��G���ð���̲�����A�����E��ˮ��Һ������ij�ֹ�ҵ������
��1��д���������ʵĻ�ѧʽ
A Y
��2��д��C�ĵ���ʽ
��3��д��A��Һ�и�����Ũ�ȵĴ�С˳���ɴ�С��
��4��д��MgCl2��D��Ӧ�Ļ�ѧ����ʽ
��5��д�����E��Һ�����ӷ���ʽ
��6����0.1molCO2ͨ��1L����ΪF����Һ�У���ַ�Ӧ��������Һ����εμ����ᣬ�����������������ɵ�����Ĺ�ϵ��ͼ��ʾ����ԭF��Һ��Ũ��Ϊ mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ӱ�ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��12�֣�Mg���仯������Է�������ת�������ַ�Ӧ��������ˮ����ȥ������֪X��Y��ZΪ��̬���ʣ�B������ΪҺ̬��������D����ɫ��ӦΪ��ɫ��C��G���ð���̲�����A�����E��ˮ��Һ������ij�ֹ�ҵ������

��1��д���������ʵĻ�ѧʽ
A Y
��2��д��C�ĵ���ʽ
��3��д��MgCl2��D��Ӧ�Ļ�ѧ����ʽ
��4������ˮ��ƽ�����۽���A+B+Mg��C+X+ MgCl2��ԭ��_______________________��
��5����0.1molCO2ͨ��1L����ΪF����Һ�У���ַ�Ӧ��������Һ����εμ����ᣬ�����������������ɵ�����Ĺ�ϵ����ͼ��ʾ����ԭF��Һ��Ũ��Ϊ mol/L��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�꽭��ʡ�����и߿���ѧ��ģ�Ծ��������棩 ���ͣ������


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com