
���� ��1��Fe2+��Fe3+���Ե������ֲ��䣬��֪�ڶ������ӷ�Ӧ��������ת��Ϊ�������ӣ�
��2����������ȡH2O2������������������Ʒ���ǿ����ȡ����ķ�Ӧ��
��3����Cl2+H2O2=2HCl+O2�У�ClԪ�صĻ��ϼ۽��ͣ�OԪ�صĻ��ϼ����ߣ�
�ڽ��ԭ�ӡ�����غ������
��4������2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2������Ϸ�Ӧ���㣮
��� �⣺��1�����������������ԣ�������Fe2+������2Fe2++H2O2+2H+�T2Fe3++2H2O�����л�ԭ�ԣ��ܱ�Fe3+��������2Fe3++H2O2=2Fe2++O2��+2H+��
�ʴ�Ϊ��2Fe3++H2O2=O2��+2Fe2++2H+��
��2��������������������Ʒ�Ӧ�����Ȼ��ƺ�H2O2������ʽΪNa2O2 +2HCl=2NaCl+H2O2��
�ʴ�Ϊ��Na2O2+2HCl�T2NaCl+H2O2��
��3����Cl2+H2O2�T2HCl+O2���÷�Ӧ��Ԫ�صĻ��ϼ۱仯Ϊ��H2O2��O2��OԪ����-1�ۡ�0�ۣ�ʧ���ӣ�����H2O2�ǻ�ԭ������ԭ������������������Ӧ��
�ʴ�Ϊ��H2O2��
����ԭ���غ�͵���غ�ã�CN-+H2O2=HCO3-+NH3�����ʴ�Ϊ��HCO3-��
��4�����ĸ�����������ʵ���Ϊ0.1mol/L��0.04L=0.004mol����˫��ˮ�����ʵ���Ϊn����
2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2��
2 5
0.004mol n
���n=0.01mol��
����˫��ˮ������Ϊ0.01mol��34g/mol=0.34g��
10.00mL���ܶȽ���Ϊ1g/mL��H2O2��Һ��Ʒ������Ϊ10g��˫��ˮ����������=$\frac{0.34g}{10g}$��100%=3.4%��
�ʴ�Ϊ��3.4%��
���� ���⿼��������ԭ��Ӧ�ļ��㣬Ϊ��Ƶ���㣬���շ�Ӧ��Ԫ�صĻ��ϼ۱仯�����ʵ�����ϵΪ���Ĺؼ������ط�������������Ŀ��飬ע���غ㷨Ӧ�ã���Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʹpH��ֽ�ʺ�ɫ����Һ�У�I-��NO3-��SO42-��Na+ | |
| B�� | ˮ�������c��H+��=1��10-13mol/L����Һ�У�CO32-��K+��ClO-��SO32- | |
| C�� | ��$\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=1��1012����Һ�У�NH4+��Ca2+��C1-��K+ | |
| D�� | ���������ܲ���������������Һ�У�NH4+��Na+��NO3-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | N2 | B�� | C2H4 | C�� | HCHO | D�� | HCN |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2-��-2-���� | B�� | 2��2-����-1-���� | ||
| C�� | 2-��-1-���� | D�� | 2��3-����-2-���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ±�ػ�������ָ��ͬ±��ԭ��֮���Թ��ۼ�����γɵĻ����xx����±�ػ�������±�ص��ʽṹ���ƣ�����������Իش��������⣺
±�ػ�������ָ��ͬ±��ԭ��֮���Թ��ۼ�����γɵĻ����xx����±�ػ�������±�ص��ʽṹ���ƣ�����������Իش��������⣺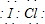 ��д����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ2NaOH+ICl�TNaCl+NaIO+H2O
��д����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ2NaOH+ICl�TNaCl+NaIO+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ʯ������裾�������裾̼���� | B�� | CI4��CBr4��CCl4��CF4 | ||
| C�� | NH3��H2O��N2��CO | D�� | �������ƣ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 13.2g | B�� | 20.1g | C�� | 22.0g | D�� | 24.0g |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com