
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ�����Լ��ڽྻ�ձ��У�������������ˮ����ֽ��裬���ã����ˣ�����Һ�ͳ����� | |
| ����2��ȡ������Һ���Թ��У��μ�ϡ���ᣮ | |
| ����3��ȡ��������1�еij������Թ��У�ȡ��������1�еij������Թ��У��μ�ϡ���ᣬ�ô��������������Թܣ��ѵ����ܲ���װ�г���ʯ��ˮ���ձ��У� | |
| ����4��ȡ������Һ���ձ��У���pH�Ʋ��� pHֵ |
���� ��1�����������ܹ��������̼��Ӧ����̼�ᱵ��
��2������3��ȡ��������1�еij������Թ��У��μ�ϡ���ᣬ�ô��������������Թܣ��ѵ����ܲ���װ�г���ʯ��ˮ���ձ��У�BaCO3�ܺ����ᷴӦ���ɵĶ�����̼������ʹʯ��ˮ����ǣ���Ԥ������ͽ��ۣ��Թ������������ɣ��ձ��еij���ʯ��ˮ����ǣ���ϲ���2˵��������BaCO3��
����4��ȡ������Һ���ձ��У���pH�Ʋ���pHֵ��Ԥ������ͽ��ۣ�pH��9.6��˵���д�����OH-���ۺ����沽���֪�Լ��ɴ���Ba��OH��2•8H2O������BaCO3��ɣ����������
��3������������һ�����ʵ���Ũ����Һ�����Ʋ���ѡ����ʵ��������ݴ˽��
������������ܶ���50ml������50mL�����������СŨ�ȣ�Ӧ����ѡ������СŨ�ȶ��ҽӽ��ģ�
����250mL��Һ������������Ũ��Ϊcmol/L���������ĵ������з��̼���c��ֵ����������250mL��Һ���������������ʵ������ݴ˼�����Ʒ��Ba��OH��2•8H2O������������
��4�����������������ܽ�ȹ��㱥����Һ�����ʵ���Ũ�ȣ��ݴ��жϣ�
��� �⣺��1���ձ���δ�����ΪBaCO3��������Ba��OH��2•8H2O��CO2����ת��ΪBaCO3��
�ʴ�Ϊ��Ba��OH��2•8H2O��CO2����ת��ΪBaCO3��
��2������3��ȡ��������1�еij������Թ��У��μ�ϡ���ᣬ�ô��������������Թܣ��ѵ����ܲ���װ�г���ʯ��ˮ���ձ��У�BaCO3�ܺ����ᷴӦ���ɵĶ�����̼������ʹʯ��ˮ����ǣ���Ԥ������ͽ��ۣ��Թ������������ɣ��ձ��еij���ʯ��ˮ����ǣ���ϲ���2˵��������BaCO3��
����4��ȡ������Һ���ձ��У���pH�Ʋ���pHֵ��Ԥ������ͽ��ۣ�pH��9.6��˵���д�����OH-���ۺ����沽���֪�Լ��ɴ���Ba��OH��2•8H2O������BaCO3��ɣ����������
�ʴ�Ϊ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ�����Լ��ڽྻ�ձ��У�������������ˮ����ֽ��裬���ã����ˣ�����Һ�ͳ����� | ---------- |
| ����2��ȡ������Һ���Թ��У��μ�ϡ���ᣮ | �а�ɫ�������ɣ�˵����Ba2+ |
| ����3��ȡ��������1�еij������Թ��У��μ�ϡ���ᣬ�ô��������������Թܣ��ѵ����ܲ���װ�г���ʯ��ˮ���ձ��� | �Թ������������ɣ��ձ��еij���ʯ��ˮ����ǣ���ϲ���2˵��������BaCO3 |
| ����4��ȡ������Һ���ձ��У���pH�Ʋ��� pHֵ | pH��9.6��˵���� ������OH-���ۺ����沽���֪�Լ��ɴ���Ba��OH��2•8H2O������BaCO3��ɣ�������� |
���� ���⿼�����ʳɷ��뺬���ⶨ����ʵ�鷽������������ơ���Һ���ơ��к͵ζ�����ѧ����ȣ���Ŀ�ۺ���ǿ���ѶȽϴ��ض�ʵ�鷽������뻯ѧ���㣮


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2O2��s����CO2��g����Ӧʱ���ų�452kJ����ʱ��ת�Ƶ�����Ϊ1.204��l023 | |
| B�� | CO��ȼ����Ϊ��H=-566kJ/mol | |
| C�� | CO��g����Na2O2 ��S����Ӧ���Ȼ�ѧ����ʽΪCO��g��+Na2O2��s���TNa2CO3��s����H=-509kJ/mol | |
| D�� | 2Na2O2��s��+2CO2��s���T2Na2CO3��s��+O2��g����H=-452kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ȼ�� | B�� | ����Һ�� | C�� | ̼��Ʒֽ� | D�� | Ũ����ϡ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ����ʳ��ˮ | B�� |  ����Ͳ��ȡҺ�� | C�� | 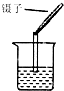 ����Һ��pH | D�� |  ����NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
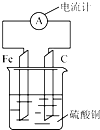 ����Ƭ��̼������ͼ��ʾ��ʽ��������ͭ��Һ�У�������ָ�뷢��ƫת��������Ը�װ�õ�˵����ȷ���ǣ�������
����Ƭ��̼������ͼ��ʾ��ʽ��������ͭ��Һ�У�������ָ�뷢��ƫת��������Ը�װ�õ�˵����ȷ���ǣ�������| A�� | ��Ƭ������ | |
| B�� | һ��ʱ���̼���������� | |
| C�� | ��װ���ܽ�����ת��Ϊ��ѧ�� | |
| D�� | ���·�е�����̼����������������������Ƭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | FeO | B�� | Fe2O3 | C�� | FeCl3 | D�� | Fe��OH��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ��Է������� | �ܶ�/��g•cm-3�� | �е�/�� | ˮ���ܽ��� | |
| ������ | 88 | 0.8123 | 131 | �� |
| ���� | 60 | 1.0492 | 118 | �� |
| ���������� | 130 | 0.8670 | 142 | ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com