
��13�֣�����ͼ��ʾ���ס������ص缫���϶���������̼������ش��������⣺
��1���������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�_____�����ҳ��е�____����
���ҳ��������ĵ缫��Ӧʽ��__ ___��
��2���������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ___ _��
�ڼ׳���̼���ϵ缫��Ӧʽ��__ ____��
�ҳ�̼���ϵ缫��Ӧ����___ ___(�������Ӧ����ԭ��Ӧ��)��
�۽�ʪ���KI������ֽ�����ҳ�̼��������������ֽ������
��Ӧ�Ļ�ѧ����ʽΪ______ __��
�����ҳ�ת��0.02 mol e-��ֹͣʵ�飬�ָ������£�������Һ�����200 mL������Һ��Ͼ��Ⱥ��PH��______��
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ��ԭ���и߶��������¿���ѧ�Ծ� ���ͣ������
��13�֣�����ͼ��ʾ���ס������ص缫���϶���������̼������ش��������⣺
��1���������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�_____�����ҳ��е�____����
���ҳ��������ĵ缫��Ӧʽ��__ ___��
��2���������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ___ _��
�ڼ׳���̼���ϵ缫��Ӧʽ��__ ____��
�ҳ�̼���ϵ缫��Ӧ����___ ___(�������Ӧ����ԭ��Ӧ��)��
�۽�ʪ���KI������ֽ�����ҳ�̼��������������ֽ������
��Ӧ�Ļ�ѧ����ʽΪ__ ____ __��
�����ҳ�ת��0.02 mol e-��ֹͣʵ�飬�ָ������£�������Һ�����200 mL������Һ��Ͼ��Ⱥ��PH��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ�߶��������¿���ѧ�Ծ� ���ͣ������
��13�֣�����ͼ��ʾ���ס������ص缫���϶���������̼������ش��������⣺
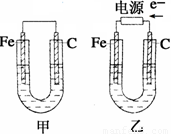
��1���������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�_____�����ҳ��е�____����
���ҳ��������ĵ缫��Ӧʽ��__ ___��
��2���������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ___ _��
�ڼ׳���̼���ϵ缫��Ӧʽ��__ ____��
�ҳ�̼���ϵ缫��Ӧ����___ ___(�������Ӧ����ԭ��Ӧ��)��
�۽�ʪ���KI������ֽ�����ҳ�̼��������������ֽ������
��Ӧ�Ļ�ѧ����ʽΪ__ ____ __��
�����ҳ�ת��0.02 mol e-��ֹͣʵ�飬�ָ������£�������Һ�����200 mL������Һ��Ͼ��Ⱥ��PH��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ֱ�ش��������⡣��10�֣�
��1����H+��Na+��Cu2+��Cl-��![]() ��ѡ������������ɵ���ʣ�������Ҫ����е��(ʹ�ö��Ե缫)��������ʵĻ�ѧʽ��գ���ֻд��һ�ּ��ɣ�
��ѡ������������ɵ���ʣ�������Ҫ����е��(ʹ�ö��Ե缫)��������ʵĻ�ѧʽ��գ���ֻд��һ�ּ��ɣ�
�ٵ���������Һ��ɫ��dz��ˮ������_______________________��
�ڵ������е���ʺ������䣬ˮ������_______________________��
�۵������У�������������������Ϊ1��1__________��
��2�����ö��Ե缫�������ͭ��Һ������������1.6��ͭ�������������ϲ���������
�ڱ�״���µ����ԼΪ ��
���ò��缫���0.02 mol/L����ͭ��Һ��ֱ��ͭ��ȫ������������Һ��������Ũ��Ϊ
��������Һ����ı仯�� ��
����֪���������Ȼ�ѧ����ʽ2H2(g)+O2(g)=2H2O(1) ��H= -571��6KJ
 C3H8(g) +5O2(g)=3CO2(g)+4H2O(1) ��H=-2220��0kJ
C3H8(g) +5O2(g)=3CO2(g)+4H2O(1) ��H=-2220��0kJ
ʵ���ã�5mol�����ͱ���Ļ��������ȫȼ��ʱ����
3847kJ����������������������������� ��
��3������ͼ��ʾ���ס������Թ��и���һö���������Թ���
ΪNaCl��Һ�����Թ�Ϊϡ������Һ�������۲쵽������
�� ������������ӦΪ
��������ӦʽΪ������������ӦΪ ��������ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͷ�ι����ձ��м���Һ�壬һ��ʱ�����װ���е��������Ե��ʹ���Һ������������Ӱ�죩���������Լ����������ǣ�����
��ͷ�ι����ձ��м���Һ�壬һ��ʱ�����װ���е��������Ե��ʹ���Һ������������Ӱ�죩���������Լ����������ǣ������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com