
| 10-9 |
| x-10-2 |
| 10-9 |
| x-10-2 |
| �ŵ� |
| ��� |
| ���� | Fe2+ | Cu2+ | Mg2+ |
| pH | 7.6 | 5.2 | 10.4 |
| c(CH3COO-)��C(H+) |
| C(CH3COOH) |
| c(CH3COO-)��C(H+) |
| C(CH3COOH) |
| x |
| 2 |
| 10-9 |
| x-10-2 |
| 10-9 |
| x-10-2 |
| ||
| ||
| 0.2L |


 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN | C6H5ONa |
| pH | 8.8 | 8.1 | 11.6 | 10.3 | 11.1 | 11.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ�����и������һ��ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
X��YΪ��������Ԫ�أ�ԭ������XС��Y��������Ԫ��Se����Ԫ��ԭ������֮��Ϊ56����֪��X������������M��N��Y��Se����������ͬ����Ԫ�أ�����Se���Դӵ�⾫��ͭ���������л�á�������������ȡSe���������£�

�ش��������⣺����Ԫ������Ӧ��Ԫ�ط��ű�ʾ��
��д��X��Y�γɵĻ�����ĵ���ʽ ��
����������Se�Ե��ʡ�Ag2Se��Cu2Se����ʽ���ڡ�д������Se������Ũ���ᷴ
Ӧ�Ļ�ѧ����ʽ
��д����Ӧ�ڵĻ�ѧ����ʽΪ ��
��ij�¶������ݻ��̶����ܱ������У����з�Ӧ�ﵽƽ�⣺
M(g)+H2O(g) N(g)+H2(g)
N(g)+H2(g)
|
n(M):n(H2O) |
Mת���� |
H2Oת���� |
|
1��1 |
0.5 |
0.5 |
|
1��2 |
0.67 |
0.335 |
|
1��3 |
0.75 |
0.25 |
����֪M��H2��ȼ���ȷֱ�Ϊ283kJ/mol��285.8kJ/mol��H2O��g��=H2O(l)��H=
��44kJ/mol��д��������Ӧ���Ȼ�ѧ����ʽ ��
�ڸ÷�Ӧ��ƽ�ⳣ��Ϊ �����¶��£��������г���1molM��3mol H2O��
2mol N��1.5molH2������ʼʱ�÷�Ӧ����V�� V�����>������<����=����
�۽�ϱ��������ж�����˵������ȷ����
A������H2O��g��������M��ת�������߶�H2O��g����ת���ʽ���
B����M��H2O��g����ת������ͬʱ�����ߵij�ʼͶ����һ����ͬ
C��M��H2O��g����ʼ���ʵ���֮�ȵ��ڶ���ת����֮��
D����M��H2O��g�����ʵ�֮��Ϊ1��4ʱ��M��ת����Ϊ0.85
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�궫��ʦ���С���ʦ���У�����ʵ����ѧ��У�����ڶ�������ģ�⿼�ԣ����� ���ͣ������
X��YΪ��������Ԫ�أ�ԭ������XС��Y��������Ԫ��Se����Ԫ��ԭ������֮��Ϊ56����֪��X������������M��N��Y��Se����������ͬ����Ԫ�أ�����Se���Դӵ�⾫��ͭ���������л�á�������������ȡSe���������£�
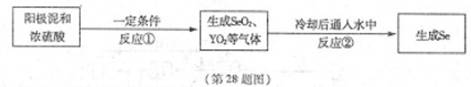
�ش��������⣺����Ԫ������Ӧ��Ԫ�ط��ű�ʾ��
��1��д��X��Y�γɵĻ�����ĵ���ʽ ��
��2����������Se�Ե��ʡ�Ag2Se��Cu2Se����ʽ���ڡ�д������Se������Ũ���ᷴӦ�Ļ�ѧ����ʽ ��
��3��д����Ӧ�ڵĻ�ѧ����ʽΪ ��
��4��ij�¶������ݻ��̶����ܱ������У����з�Ӧ�ﵽƽ�⣺M(g)+H2O(g) N(g)+H2(g)
N(g)+H2(g)
|
n(M):n(H2O) |
Mת���� |
H2Oת���� |
|
1��1 |
0.5 |
0.5 |
|
1��2 |
0.67 |
0.335 |
|
1��3 |
0.75 |
0.25 |
����֪M��H2��ȼ���ȷֱ�Ϊ283kJ/mol��285.8kJ/mol��H2O��g��=H2O(l)��H=��44kJ/mol��
д��������Ӧ���Ȼ�ѧ����ʽ ��
�ڸ÷�Ӧ��ƽ�ⳣ��Ϊ �����¶��£��������г���1molM��3mol H2O��2mol N��1.5molH2������ʼʱ�÷�Ӧ����V�� V�����>������<����=����
�۽�ϱ��������ж�����˵������ȷ����
A������H2O��g��������M��ת�������߶�H2O��g����ת���ʽ���
B����M��H2O��g����ת������ͬʱ�����ߵij�ʼͶ����һ����ͬ
C��M��H2O��g����ʼ���ʵ���֮�ȵ��ڶ���ת����֮��
D����M��H2O��g�����ʵ�֮��Ϊ1��4ʱ��M��ת����Ϊ0.85
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN | C6H5ONa |
| pH | 8.8 | 8.1 | 11.6 | 10.3 | 11.1 | 11.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ��У�߶����ϣ���ĩ��ѧ�Ծ���������ѧ�����һ�С�����һ�С��人���У��������棩 ���ͣ������
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN | C6H5ONa |
| pH | 8.8 | 8.1 | 11.6 | 10.3 | 11.1 | 11.3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com