


 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д� ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
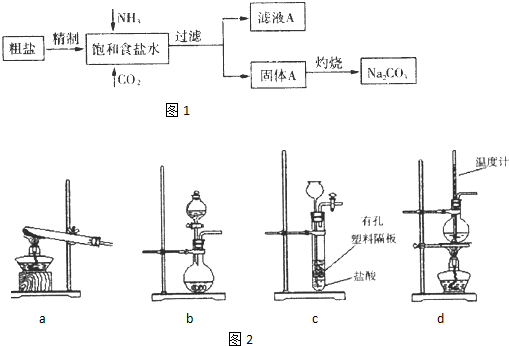
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����һ����ļ�����Һ�������Ӽ���ʱ�ó��Ľ����Ǻ��У�AlO2-��Fe3+��HCO3- | B��ijһ��������ˮ���������K+��NO3-��Na+��Cl-�����ӣ���ù���������KNO3��NaCl�Ļ�����KCl��NaNO3�Ļ���� | C������NaOH�����˿����еĶ�����̼������ˮ������Һ�к��϶��HCO3- | D��ij��Һ�м���BaCl2���ֲ�����ϡHNO3�İ�ɫ�����������Һ��һ������SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������Һ��һ��������Ba2+��HCO3- | B��ȡ������Һ����KSCN����Һ�Ժ�ɫ����ԭ��Һ��һ����Fe3+ | C������Һ��һ������SO42-��Cl- | D����ȡ����Һ���ٹ�NaOH��Һ���ȣ��Թܿڵ�ʪ���ɫʯ����ֽ����������ԭ ��Һ��һ��������NH4+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һƿ����ļ�����Һ�������Ӽ���ʱ�ó��Ľ����Ǻ���Ca2+��Fe2+��Na+��Cl-��![]()
��ijһ������������ˮ�������K+��![]() ��Na+��Cl-����ù���������KNO3��NaCl��NaNO3��KCl�Ļ����
��Na+��Cl-����ù���������KNO3��NaCl��NaNO3��KCl�Ļ����
�۹���NaOH�������˿����е�CO2������ˮ����Һ�лẬ�н϶��![]()
���Ȼ�����Һ�����ԣ�ij����ʹ���Ϊ������Һ�� �������м����ռ���Һ
A.�ٺ͢� B.�ں͢� C.�ں͢� D.�٢ۢ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com