
 BaCO3”ż£«NH3”ü£«2H2O
BaCO3”ż£«NH3”ü£«2H2O


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| c(H+) |
| c(OH-) |
| A£®¢Ś¢Ż | B£®¢Ū¢Ż | C£®¢Ś¢Ū¢Ü | D£®¢Ł¢Ü¢Ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
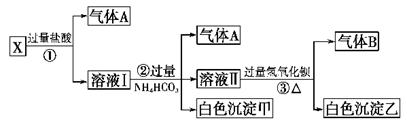
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĀČ»ÆĀĮČÜŅŗÖŠ¼ÓČė¹żĮæ°±Ė®£ŗAl3£«£«4NH3”¤H2O=AlO2”Ŗ£«4NH4+£«2H2O |
| B£®ÅØÉÕ¼īČÜŅŗÖŠ¼ÓČėĀĮʬ£ŗAl£«2OH£=AlO2”Ŗ£«H2”ü |
| C£®ÓĆÅØNaOHČÜŅŗČܽāAl2O3£ŗ2OH££«Al2O3=2AlO2”Ŗ£«H2O |
| D£®ĶłĢ¼ĖįĆ¾ÖŠµĪ¼ÓĻ”ŃĪĖį£ŗCO32”Ŗ£«2H£«=CO2”ü£«H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®K£«Ņ»¶Ø“ęŌŚ |
| B£®100 mLČÜŅŗÖŠŗ¬0.01 mol CO32”Ŗ |
| C£®Cl£æÉÄÜ“ęŌŚ |
| D£®Ba2£«Ņ»¶Ø²»“ęŌŚ£¬Mg2£«æÉÄÜ“ęŌŚ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®NH4+ NO3- CO32- Na£« |
| B£®Na£«Ba2£«Mg2£«HCO3- |
| C£®NO3- K£«AlO2- OH£ |
| D£®NO3- Ca2£«K£«Cl£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĒŠæŖµÄ½šŹōNa±©Ā¶ŌŚæÕĘųÖŠ£¬¹āĮĮ±ķĆęÖš½„±ä°µ2Na£«O2=Na2O2 |
| B£®ĻņAgClŠü×ĒŅŗÖŠµĪ¼ÓNa2SČÜŅŗ£¬°×É«³Įµķ±ä³ÉŗŚÉ«2AgCl£«S2£=Ag2S”ż£«2Cl£ |
| C£®Na2O2ŌŚ³±ŹŖµÄæÕĘųÖŠ·ÅÖĆŅ»¶ĪŹ±¼ä£¬±ä³É°×É«Õ³³ķĪļ2Na2O2£«2CO2=2Na2CO3£«O2 |
| D£®ĻņNaHCO3ČÜŅŗÖŠ¼ÓČė¹żĮæµÄ³ĪĒåŹÆ»ŅĖ®£¬³öĻÖ°×É«³Įµķ2HCO3-£«Ca2£«£«2OH£=CaCO3”ż£«CO32-£«2H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®K£«Ņ»¶Ø“ęŌŚ | B£®100mLČÜŅŗÖŠŗ¬0.01mol CO32£ |
| C£®Cl£Ņ»¶Ø“ęŌŚ | D£®Ba2£«Ņ»¶Ø²»“ęŌŚ£¬Mg2£«æÉÄÜ“ęŌŚ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com