
���� ��1����ȡ8.70g��ʯ����������400�����ʧȥȫ���ᾧˮ��������Ϊ8.16gΪ��������ʵ���=$\frac{8.16g}{136g/mol}$=0.06mol�����ٵ�����Ϊʧȥ�Ľᾧˮ���������ݴ˼���õ������нᾧˮ������������
��2���ٳ�ȡ8.70g�ĸ�ʯ������������80%�ľƾ���Һ��ʹCaSO4��ȫˮ����CaSO4•$\frac{1}{2}$H2O����ʱ�������������ƾ���Һ�������ã�������ȡ����������������������Ϊ8.97g��CaSO4+$\frac{1}{2}$H2O=CaSO4•$\frac{1}{2}$H2O�����ӵ�$\frac{1}{2}$H2O����=8.97g-8.7g=0.27g����$\frac{1}{2}$H2O���ʵ���=$\frac{0.27}{18g/mol}$=0.015mol��CaSO4���ʵ���=0.03mol��
��3���ڢ�������8.97g�����м�������ˮ��ʹȫ����CaSO4•$\frac{1}{2}$H2O��ˮ����CaSO4•2H2O��ȡ��������ٴγ�����������Ϊ10.32g��CaSO4•$\frac{1}{2}$H2O+$\frac{3}{2}$H2O=CaSO4•2H2O����������Ϊ$\frac{3}{2}$H2O����=10.32g-8.97g=1.35g�����ʵ���=$\frac{1.35g}{18g/mol}$=0.075mol����CaSO4•$\frac{1}{2}$H2O���ʵ���Ϊ0.05mol��ԭ������CaSO4•2H2O���ʵ���=0.06mol-0.05mol=0.01mol��
��� �⣺��1����ȡ8.70g��ʯ����������400�����ʧȥȫ���ᾧˮ��������Ϊ8.16gΪ��������ʵ���=$\frac{8.16g}{136g/mol}$=0.06mol�����ٵ�����Ϊʧȥ�Ľᾧˮ���������ݴ˼���õ������нᾧˮ����������=$\frac{8.7g-8.16g}{8.7g}$=0.062��
�ʴ�Ϊ��0.062��
��2���ٳ�ȡ8.70g�ĸ�ʯ������������80%�ľƾ���Һ��ʹCaSO4��ȫˮ����CaSO4•$\frac{1}{2}$H2O����ʱ�������������ƾ���Һ�������ã�������ȡ����������������������Ϊ8.97g��CaSO4+$\frac{1}{2}$H2O=CaSO4•$\frac{1}{2}$H2O�����ӵ�$\frac{1}{2}$H2O����=8.97g-8.7g=0.27g����$\frac{1}{2}$H2O���ʵ���=$\frac{0.27}{18g/mol}$=0.015mol��CaSO4���ʵ���=0.03mol��
�ʴ�Ϊ��0.03��
��3���ڢ�������8.97g�����м�������ˮ��ʹȫ����CaSO4•$\frac{1}{2}$H2O��ˮ����CaSO4•2H2O��ȡ��������ٴγ�����������Ϊ10.32g��CaSO4•$\frac{1}{2}$H2O+$\frac{3}{2}$H2O=CaSO4•2H2O����������Ϊ$\frac{3}{2}$H2O����=10.32g-8.97g=1.35g�����ʵ���=$\frac{1.35g}{18g/mol}$=0.075mol����CaSO4•$\frac{1}{2}$H2O���ʵ���Ϊ0.05mol��ԭ������CaSO4•2H2O���ʵ���=0.06mol-0.05mol=0.01mol��ԭ��Ʒ��CaSO4•$\frac{1}{2}$H2O���ʵ���=0.05mol-0.03mol=0.02mol��������������ʯ������ʵ���֮��Ϊn��CaSO4•2H2O����n��CaSO4•$\frac{1}{2}$H2O����n��CaSO4��=1��2��3��
�ʴ�Ϊ��1��2��3��
���� ���⿼���˽ᾧˮ����ļ��㣬��Ҫ�������仯�Ķ�������Ӧ�ã�ע������������仯��ʵ�����⣬���ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�


 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ֤��һƿ����ɫ���������������Ƕ�������������ʪ��ĵ⻯��һ������ֽ���飬�۲���ֽ��ɫ�ı仯 | |
| B�� | ���ȼ���������ϡ�����ٵμ�KSCN��Һ��δ����Ѫ��ɫ�������ȼ����������� | |
| C�� | ����ˮ��pH���ò�����պȡ��ˮ����pH��ֽ�ϣ������ɫ��ͱ���ɫ���Ƚ� | |
| D�� | ���������Һ�У��������ᣬ������֤S�ķǽ�����ǿ��Si |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ����� | �� | �� | �� |
| �Ͻ�����/g | 8.8 | 17.6 | 22.0 |
| �������/L | 2.24 | 4.48 | 4.48 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 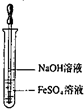 ���������������� | B�� |  ���ȷ�Ӧ | ||
| C�� | 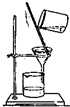 ��ȥ�����ܽ��е�NaCl | D�� | 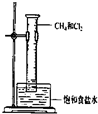 �����ȡ����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
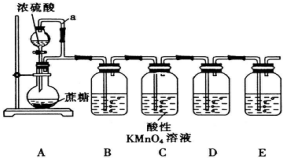
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
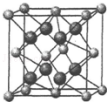 ������Ȼ���г���CaF2����ʽ���ڣ�
������Ȼ���г���CaF2����ʽ���ڣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com