������A��B����ѧ���������ʣ����������ӿɴ��±���ѡ��
| ������ |
K+ Na+ Fe2+ Ba2+ NH4+ |
| ������ |
OH- NO3- I- HCO3- AlO2- HSO4- |
��1����A��B��ˮ��Һ��Ϊ��ɫ��B��ˮ��Һ�ʼ��ԣ��һ�Ϻ�ֻ����������ϡ����İ�ɫ��������ʹ��ɫʯ����ֽ���������壮
��B�Ļ�ѧʽΪ
Ba��OH��2
Ba��OH��2
��
��A��B��Һ��Ϻ���ȳ����Է�Ӧ�����ӷ���ʽΪ
H
++SO
42-+NH
4++Ba
2++2OH
-BaSO
4��+NH
3��+2H
2O
H
++SO
42-+NH
4++Ba
2++2OH
-BaSO
4��+NH
3��+2H
2O
��2����A��ˮ��Һ��dz��ɫ��B��ˮ��Һ��ɫ������ɫ��ӦΪ��ɫ����A��ˮ��Һ�м���ϡ���������������ټ���B����Һ��ƣ���A��B��ˮ��Һ����������Ա仯�����AΪ
FeI2
FeI2
��BΪ
NaNO3
NaNO3
��
�ھ�����������������Һ��Ƶ�ԭ������������֣���
����I-��������I2ʹ��Һ�ʻ�ɫ
����I-��������I2ʹ��Һ�ʻ�ɫ
��
I-��Fe2+��������ʹ��Һ�ʻ�ɫ
I-��Fe2+��������ʹ��Һ�ʻ�ɫ
��
������һ������֤��������Һ��Ƶ�ԭ��
ȡ���������Һ���Թ��У��μӼ���KSCN��Һ�������������
ȡ���������Һ���Թ��У��μӼ���KSCN��Һ�������������
��
��������Һ���ԭ����������Ƴ�ԭ��أ�����1.8mol������a����b����b���ĵ缫��ӦʽΪ
NO3-+4H++3e-�TNO��+2H2O
NO3-+4H++3e-�TNO��+2H2O
����a��b��Ϊʯī�缫��b��
��ԭ
��ԭ
�����������ԭ������������ʵ�����
0.6mol
0.6mol
��



 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
 ��ѧ��ѧ�г�������������ͼ��ʾ��ת����ϵ����Ӧ������ȥ����X��Y��Z��W�ǵ��ʣ������Ϊ�����A��W��Z�����³���̬����A��һ�ִ�����Ⱦ�B��һ�ֳ��õ��ᣮ
��ѧ��ѧ�г�������������ͼ��ʾ��ת����ϵ����Ӧ������ȥ����X��Y��Z��W�ǵ��ʣ������Ϊ�����A��W��Z�����³���̬����A��һ�ִ�����Ⱦ�B��һ�ֳ��õ��ᣮ


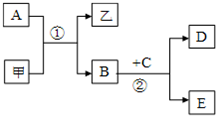 ͼ��ʾ��ת����ϵ�У��ס��Ҽ�A��B��C��D��E��Ϊ��ѧ�����Ļ�ѧ���ʣ����мס���Ϊ�����A��B��DΪ���ʣ�
ͼ��ʾ��ת����ϵ�У��ס��Ҽ�A��B��C��D��E��Ϊ��ѧ�����Ļ�ѧ���ʣ����мס���Ϊ�����A��B��DΪ���ʣ� A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺ A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
