
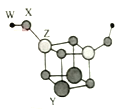
����Ŀ������������Ԫ��W��X��Y��Z��ԭ������������������Ԫ�ؿ������һ�ַ��Ӵأ�����ӽṹ��ͼ��ʾ�������С��ʾԭ�Ӱ뾶����Դ�С����W��Xλ�ڲ�ͬ���ڣ�Xԭ�ӵ������������Ǵ�����������3����Z��������������������������˵������ȷ����
A.Y��Z�ļ����Ӿ���Ӱ��ˮ�ĵ���ƽ��
B.��ҵ�ϻ��Y��Z���ʾ��ɲ��õ�����ǵ������Ȼ���
C.Y������������Ӧˮ����ļ��Ա�Z����
D.��ͬ�����£�W���ʵķе��X���ʵĵ�
���𰸡�D
��������
������Xԭ�ӵ������������Ǵ�����������3������֪XΪO������ͼʾ��֪ԭ�Ӱ뾶��Y>Z>X>W����Z��������������������������ZΪAl�� W��Xλ�ڲ�ͬ���ڣ�����WΪH��Y��ԭ��������ZС���뾶��Z��Y��Zͬ���ڣ�����ΪNa��Mg��
A.ZΪAl��Al3+��ˮ��Һ�з���ˮ��Ӱ��ˮ�ĵ���ƽ�⣬��A����
B. ZΪAl�����Ȼ���Ϊ�Ȼ������Ȼ���Ϊ���ۻ��������״̬�²����磬���ܵ����ȡ�����ʣ���B����
C.YΪ�ƻ�þ�������Ա���ǿ������������Ӧˮ����ļ��Ա�����ǿ����C����
D.WΪ�⣬�䵥��Ϊ������XΪ������Ӧ����Ϊ�������������Ϊ���Ӿ��壬��ͬ�����£��۷е�����Է��������йأ��������������Է����������������������۷е�ߣ���D��ȷ��
�ʴ�ΪD��


 ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ԫ��X��Y��Z��Wλ��Ԫ�����ڱ���ǰ�����ڣ���֪���ǵĺ˵�����������ӣ��Һ˵����֮��Ϊ51��Yԭ�ӵ�L��p�������2�����ӣ�Z��Yԭ�ӵļ۲��������ͬ��Wԭ�ӵ�L�������������������֮��Ϊ4��1����d����еĵ�����������������֮��Ϊ5��1��
(1)Y��Z�ɷֱ���X�γ�ֻ��һ������ԭ�ӵĹ��ۻ�����a��b�����ǵķ���ʽ�ֱ���________��________���ӻ�����ֱ���________��________��a���ӵ�����ṹ��________��
(2)Y������������Z�����������ľ������ͷֱ���________���塢________���塣
(3)Y��Z�Ƚϣ��縺�Խϴ����________��W2�����ӵĺ�������Ų�ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ˮԡ���ȵμӷ�̪��NaHCO3��Һ����ɫ��pH���¶ȱ仯���£�����ˮ�Ļӷ�����
ʱ�� | �� | �� | �� | �� | �� |
�¶�/�� | 20 | 30 | 40 | ��40����ȴ��20�� | ��ˮԡ����ȴ��20�� |
��ɫ�仯 | ��ɫ�Լ��� | ��ɫ�ӽ��� | ��ɫ�Ȣۼ���϶� | ||
pH | 8.31 | 8.29 | 8.26 | 8.31 | 9.20 |
����˵������ȷ���ǣ� ��
A.NaHCO3��Һ�Լ��Ե�ԭ��HCO3-+H2O![]() H2CO3+OH-
H2CO3+OH-
B.�����۵Ĺ����У���ɫ�����ԭ�������HCO3-ˮ��̶�����
C.�����۵Ĺ����У�pH���½�˵�����¹�����c(OH-)��С
D.�ݱȢ�pH�����Ʋ�������NaHCO3�ֽ����ɵ�Na2CO3��Ե��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ̽��ij�л�������A�Ľṹ�����ʣ���������ʵ�飺
I.ȷ������ʽ
��1�����л���A�����������г��ȼ��ʵ���ã�����5.4gH2O��8.8gCO2����������6.72L(��״����)����A�и�Ԫ�ص�ԭ�Ӹ�����Ϊ______________��
��2��A������ͼ��ͼ1��ʾ����A�ķ���ʽΪ_____________��
II.�ṹʽ��ȷ��
��3�����ⶨ��A�ĺ˴Ź�������ͼ��ͼ2��ʾ����A�Ľṹ��ʽΪ____________��
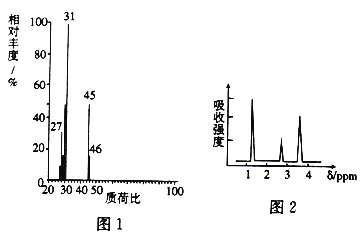
III.����ʵ��
��4��A��һ�������¿���ˮ������ɫ����B���÷�Ӧ�Ļ�ѧ����ʽΪ____________��
��5�����������е��˶�Ա����Ť��ʱ����ҽ�漴��������(�е�Ϊ12.27��)�����˲�λ���оֲ��䶳�������Ʊ��������һ���÷�������A��SOCl2�����·�Ӧ��ͬʱ���ɶ���������Ȼ����������壬��÷�Ӧ�Ļ�ѧ����ʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ����������ϩ����ȫȼ��ʱ�ų�������ΪQ����ȫ����ȼ�պ������ɵ�CO2������Ҫ200mL 2mol/L��NaOH��Һ����28g��ϩ��ȫȼ�շų���������������
A. 5QB. 5Q��10QC. 10QD. ����10Q
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʱ������һ��c(H2A)��c(HA��)��c(A2��)��0.10mol��L��1��H2A��NaOH�Ļ����Һ����Һ�в����������ʵ���Ũ����pH�ı仯������ͼ��ʾ������˵��������ȷ����
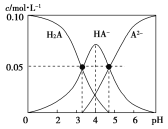
A.��c(Na��)��0.10mol��L��1����Һ�У�c(H2A)��c(OH��)��c(A2��)��c(H��)
B.��pH=4����Һ��, c(HA-)��c(A2��) + c(H2A)
C.��pH��7����Һ�У�c(Na��)��2c(A2��)
D.K1(H2A)��������ԼΪ10-3.3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ֶ�����Ԫ�������ڱ��е����λ��������ʾ������ZԪ��ԭ�Ӻ��������������������������3����
X | Y | |
Z | W |
��ش��������⣺
��1��Ԫ��Zλ�����ڱ��еĵ�_________���ڣ�_________�壻
��2����ЩԪ�ص��⻯���У�ˮ��Һ������ǿ����_______________��д��ѧʽ����
��3��XW2�ĵ���ʽΪ_______________��
��4��Y�����������Ļ�ѧʽΪ________________��
��5��W��Y�γɵ�һ�ֶ�Ԫ���������ɫ��ЧӦ����Է���������170��190֮�䣬��W����������ԼΪ70�����û�����Ļ�ѧʽΪ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ��ͨ��ʵ��̽�����̼�õ���������ǿ����ͨ���Ƚ���������������Ӧˮ���������ǿ������֤���������ͼʵ�顣
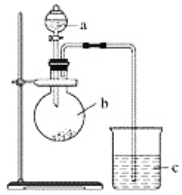
��1������a��������________��Ӧʢ������ҩƷ�е�________(����ĸ)��
A��ϡ���� B��������C�������� D������
��2������b��������________��Ӧʢ������ҩƷ�е�________(����ĸ)��
A��̼��� B�������� C���Ȼ��� D��̼����
��3������c��Ӧʢ�ŵ��Լ���________�����������������____________________��֤��b�з�Ӧ������________������֤��________��________����ǿ���õ�������________��________ǿ��b�з�����Ӧ�����ӷ���ʽΪ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������µ��ܱ������У�4NH3(g)��5O2(g)![]() 4NO(g)��6H2O(g) ��H����905.9kJ��mol��1������������ȷ���ǣ� ��
4NO(g)��6H2O(g) ��H����905.9kJ��mol��1������������ȷ���ǣ� ��
A.4molNH3��5molO2��Ӧ���ﵽƽ��ʱ�ų�����Ϊ905.9kJ
B.ƽ��ʱv��(O2)��![]() v��(NO)
v��(NO)
C.ƽ���ѹǿ���������ƽ��Ħ����������
D.ƽ��������¶ȣ����������NO��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com