
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �������ʵ�飺������ͼ1��ʾ��װ����ȡ�����飻�ڽ��������������ʵ�飮���Թ�I�����μ���2mL ����ˮ��4mLŨ���ᡢ2mL 95%���Ҵ���3g�廯�Ʒ�ĩ�����Թܢ���ע������ˮ�����ձ���ע������ˮ�������Թ�I����״̬�����Ӻ���ȴ���Իش��������⣺
�������ʵ�飺������ͼ1��ʾ��װ����ȡ�����飻�ڽ��������������ʵ�飮���Թ�I�����μ���2mL ����ˮ��4mLŨ���ᡢ2mL 95%���Ҵ���3g�廯�Ʒ�ĩ�����Թܢ���ע������ˮ�����ձ���ע������ˮ�������Թ�I����״̬�����Ӻ���ȴ���Իش��������⣺| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A_______________��B_______________��C_______________��D_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
д��4���л��������ȫȼ�ղ���CO2��H2O����̬��������Ⱦ�Ϊ1��1�����ܱ����Ƶ�Cu(OH)2�������л���Ľṹ��ʽ��
A_______________��B_______________��C_______________��D_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ�����и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
�������ʵ�飺
����ͼ��ʾ��װ����ȡ�����飻�ڽ��������������ʵ�顣���Թ�I�����μ���2 mL ����ˮ��4 mLŨ���ᡢ2 mL 95�����Ҵ���3g�廯�Ʒ�ĩ�����Թܢ���ע������ˮ�����ձ���ע������ˮ�������Թ�I����״̬�����Ӻ���ȴ���Իش��������⣺

��1���Թ�I��Ũ�������廯�Ƽ��ȷ�Ӧ���������ᣬд�����������Ҵ��ڼ���ʱ��Ӧ�Ļ�ѧ����ʽ _��
��2���Թ�I�з�Ӧ�������������飬���������ɵ��л����� _ �� (д�������л���Ľṹ��ʽ)��
��3��������ķе�ϵͣ��ӷ���Ϊ��ʹ�������������Թܢ��У����ٻӷ�����ͼ�в�ȡ�Ĵ�ʩ�� �� _��
��4���ڽ�����������NaOH�Ҵ���Һ���ȵ�����ʵ��ʱ�������ɵ�����ͨ����ͼ��ʾ��װ�á�����ͼװ�ý���ʵ���Ŀ���� _����ͼ���ұ��Թ��е������� ��ˮ�������� ��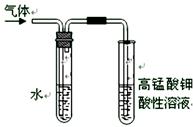
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com