
����Ŀ�����ݻ�Ϊ2L���ܱ������н������·�Ӧ��
A��g��+2B��g��![]() 3C��g��+nD��g������ʼʱAΪ4mol��BΪ6mol��5minĩʱ���C�����ʵ���Ϊ3mol����D��ʾ�Ļ�ѧ��Ӧ����v��D��Ϊ0.2mol/��L��min��������
3C��g��+nD��g������ʼʱAΪ4mol��BΪ6mol��5minĩʱ���C�����ʵ���Ϊ3mol����D��ʾ�Ļ�ѧ��Ӧ����v��D��Ϊ0.2mol/��L��min��������
��1��5minĩA�����ʵ���Ũ��Ϊ__________ mol/L��
��2��ǰ5min����B��ʾ�Ļ�ѧ��Ӧ����v��B��Ϊ___________ mol/��L��min����
��3����ѧ����ʽ��nֵΪ__________��
��4���˷�Ӧ�����ֲ�ͬ����µķ�Ӧ���ʷֱ�Ϊ��
��v��A��=5mol/��L��min��
��v��B��=6mol/��L��min��
��v��C��=4.5mol/��L��min��
��v��D��=8mol/��L��min��
���з�Ӧ����������__________�����ţ���
���𰸡�![]()
�������������������1������C�����ʵ���Ϊ3mol��������A�����ʵ���Ϊ1mol�����5minĩA��c(A)=(4��1)/2mol��L��1=1.5mol��L��1����2������C�����ʵ���n(C)=3mol��������n(B)=2mol��v(B)="2/(2��5)mol/(L��min)=0.2" mol/(L��min)����3�����ݻ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ�v(B)��v(D)=0.2��0.2=1��1����n=2����4����AΪ������v(A)=v(B)/2=3mol/(L��min)����v(A)=c(C)/3=1.5mol/(L��min)����v(A)=v(D)/2=4mol/(L��min)����˷�Ӧ��������������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��ȷ���Ƽ�����[KFe3(SO4)x(OH)y]�Ļ�ѧʽ��ij��ȤС�����������ʵ�飺

��ش�
(1)�Ƽ������Ļ�ѧʽ[KFe3(SO4)x(OH)y]��x=________��y=________��
(2)д����ҺB���������ʵĻ�ѧʽ________��
(3)���ɫ����������HI��Һ��������������ԭ��Ӧ��д���÷�Ӧ�����ӷ���ʽ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ���ѧ����������ᡢ����������ء������й�˵������ȷ����(�� ��)
A. ����ȼ�ŵ������ijЩ����Ԫ����ɫ��Ӧ�����ֳ�����ɫ��
B. Ϊ��ֹ�����±��ȸ�֬ʳƷ�����������ʣ����ڰ�װ���з�����ʯ��
C. ��ѧҩƷ�Ż𣬶�Ҫ������ˮ����ĭ��������
D. PM2.5�к���Ǧ������������ȶ������к��Ľ���Ԫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ƿ�չ�е�����Դ���������ð�������Ʊ��������Ӧ���������ڡ��ش��������⣺
��1����������ȣ�������Ϊȼ�ϵ��ŵ���_________�����ٴ�����㣩����������ֱ��ȼ�յ�����ת����Զ����ȼ�ϵ�أ�д����������ȼ�ϵ�صĸ�����Ӧʽ��____________��
��2���Լ���Ϊԭ�Ͽ��Ƶ�������ͼ1��һ���¶ȡ�ѹǿ�£�CH4(g)��H2O(g)��Ӧ����CO(g)��1mol H2(g)��������KJ���仯ʾ��ͼ��д���÷�Ӧ���Ȼ�ѧ����ʽ_______________(��H��E1��E2��E3��ʾ)��

��3���ں��º��ݵ��ܱ������У�ij���ⷴӦ��MHx(s)+yH2(g)![]() MHx+2y(s) ��H<0�ﵽ��ѧƽ�⡣�����й�������ȷ����________��
MHx+2y(s) ��H<0�ﵽ��ѧƽ�⡣�����й�������ȷ����________��
A������������ѹǿ���ֲ���
B������y mol H2ֻ��1 mol MHx
C�������£��÷�Ӧ��ƽ�ⳣ������
D������������ͨ��������������v(����)>v(����)
��4�����������ĸ�����Ҳ����������Դ����ⷨ��ȡ�й㷺��;��Na2FeO4��ͬʱ���������Fe+2H2O+2OH-![]() FeO42-+3H2��������ԭ����ͼ1��ʾ��װ��ͨ������缫���������Ϻ�ɫ��FeO42-�����缫�����ݲ�����������������ҺŨ�ȹ��ߣ����缫����������ɫ���ʡ���֪��Na2FeO4ֻ��ǿ�����������ȶ����ױ�H2��ԭ��
FeO42-+3H2��������ԭ����ͼ1��ʾ��װ��ͨ������缫���������Ϻ�ɫ��FeO42-�����缫�����ݲ�����������������ҺŨ�ȹ��ߣ����缫����������ɫ���ʡ���֪��Na2FeO4ֻ��ǿ�����������ȶ����ױ�H2��ԭ��

�ٵ��һ��ʱ���c(OH-)���͵�������__________(������ҡ��������ҡ�)��
�ڵ������У��뽫�������������弰ʱ�ų�����ԭ����_____________��
��c(Na2FeO4)���ʼc(NaOH)�ı仯��ͼ2������N��c( Na2FeO4)�������ֵ��ԭ��_____________��
��5�����ݻ��ɱ���ܱ������г���10molCO��20molH2������CO ( g ) + 2H2 ( g )![]() CH3OH ( g ) ��H<0, CO ��ƽ��ת�������¶ȣ�T����ѹǿ��P���ı仯��ͼ��ʾ�����ﵽƽ��״̬A ʱ�����������Ϊ1 L������Ӧ��ʼʱ�Գ���10mol CO ��20mol H2������ƽ��״̬Bʱ���������V��B��= L��
CH3OH ( g ) ��H<0, CO ��ƽ��ת�������¶ȣ�T����ѹǿ��P���ı仯��ͼ��ʾ�����ﵽƽ��״̬A ʱ�����������Ϊ1 L������Ӧ��ʼʱ�Գ���10mol CO ��20mol H2������ƽ��״̬Bʱ���������V��B��= L��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȼ�ϵ�ؾ��й����ķ�չǰ;����ѧ�ҽ������Ƴ�һ���͵�ȼ�ϵ�أ����ü״�ȡ��������ȼ�Ͽ��Լ����ƣ��õ������ȡ����ͳ��أ�ijѧ����ʵ�������ü��Լ״�ȼ�ϵ�ص��Na2SO4��Һ��
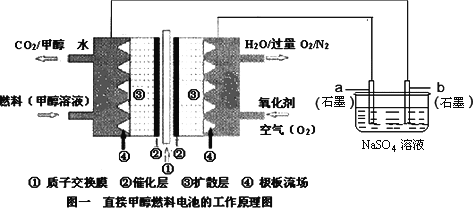
�����ͼʾ�ش��������⣺
��1��ͼ��a�缫��_____________(�����������������������������������)���õ缫�Ϸ����ĵ缫��ӦʽΪ__________________��
��2�����������£�ͨ��״���һ�������ĵ缫��ӦʽΪ________________��
��3��������3.36 L����ʱ(���ۺ�Ϊ��״��)�������ϵ��Na2SO4��Һ��������������ʵ�����____________mol��
��4����a��b�缫���Ϸֱ�Ϊ����ʯī�������ܷ�Ӧ��ѧ����ʽΪ____________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵���д�����ǣ� ��
A. COȼ���Ƿ��ȷ�Ӧ B. H2SO4��NaOH��Ӧ�Ƿ��ȷ�Ӧ
C. CaO��H2O��Ӧ�����ȷ�Ӧ D. CaCO3���ȷֽ������ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�ȡ0.3 mol/L HY��Һ��0.3 mol/L NaOH��Һ�������ϣ����ƻ�Ϻ���Һ����仯������û����Һ��pH=9��������˵�������ϵʽ����ȷ����
A. �����Һ����ˮ���������c(OH-)=1��10-9 mol/L
B. �����Һ������Ũ�ȴ�С����Ϊ��c(Y-)>c(Na+)>c(OH-)>c(H+)
C. c(OH-)-c(HY)=c(H+)=1��10-9 mol/L
D. c(Na+)=c(Y-)+c(HY)="0.3" mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ij�¶���CH3COOH�ĵ��볣��K=1.6��10-5�����¶��£���20ml 0.01mol/L CH3COOH��Һ����μ���0.01mol/L KOH��Һ����pH�仯������ͼ��ʾ(�����¶ȱ仯)����ش������й����⣺

��1��a����Һ��c(H+)Ϊ ��pHԼΪ ��
��2�� a��b��c��d�ĵ���ˮ�ĵ���̶������� �㣬�ζ���������ѡ�� ��ָʾ�����ζ��յ��� ���c�����ϡ���c�����¡�����
��3�����в����п���ʹ����CH3COOH��Һ��Ũ����ֵƫ�͵��� ������ĸ����
A����ʽ�ζ���δ�ô�����Һ��ϴ
B����ƿ�ô�����Һ��ϴ
C���ų���Һ�ĵζ��ܿ�ʼ�����ݣ��ų�Һ���������ʧ
D����ʽ�ζ��ܵζ�ǰ�����ݣ��ζ��յ�ʱ������ʧ
E.��ʽ�ζ��ܵζ�ǰ������ȷ���ζ����Ӷ���
��4������20mlϡ��ˮ����μ����Ũ�ȵ����ᣬ���б仯������ȷ���� ������ţ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����4�ֻ����Һ���ֱ��ɵ����0.1 mol��L��1��������Һ��϶��ɣ���NH4Cl��CH3COONa����NH4Cl��HCl����NH4Cl��NaCl����NH4Cl��NH3��H2O�����и���������ȷ����
A. pH����<��<��<�� B. ��Һ��c(H��)����<��<��<��
C. c(NH![]() )����<��<��<�� D. c(NH3��H2O)����<��<��<��
)����<��<��<�� D. c(NH3��H2O)����<��<��<��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com