
 Š“³öľĢæÓėÅØĮņĖį·“Ó¦µÄ»Æѧ·½³ĢŹ½
Š“³öľĢæÓėÅØĮņĖį·“Ó¦µÄ»Æѧ·½³ĢŹ½
| ||
| ||
| ||
| ||



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø11·Ö£©
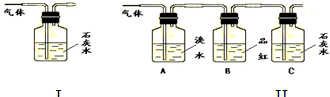
¢ń£®¼×Ķ¬Ń§ĄūÓĆĻĀĮŠ×°ÖĆŃé֤ľĢæÓėÅØĮņĖį·“Ó¦µÄČ«²æ²śĪļ
£Ø1£©Š“³öľĢæÓėÅØĮņĖį·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
£Ø2£©AÖŠ¼ÓČėµÄŹŌ¼ĮŹĒ £¬B”¢DÖŠ¼ÓČėµÄŹŌ¼Į¶¼ŹĒĘ·ŗģČÜŅŗ£¬DÖŠÕżČ·µÄŹµŃéĻÖĻóŹĒ£ŗ
£Ø3£©ŹµŃ鏱£¬CÖŠČō¼ÓČėµÄŹĒµĪÓŠµķ·ŪµÄµāĖ®£¬¹Ū²ģµ½µÄĻÖĻóŹĒ £¬
Ąė×Ó·½³ĢŹ½ĪŖ£ŗ ”£
¢ņ£®ŅŅĶ¬Ń§Ö»ÓĆB”¢C”¢D”¢E×°ÖĆŃéÖ¤SO2µÄijŠ©ŠŌÖŹ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©CÖŠ¼ÓČėµÄŹŌ¼ĮŹĒ £¬Ö¤Ć÷SO2¾ßÓŠŃõ»ÆŠŌ”£
£Ø2£©DÖŠ¼ÓČėĖįŠŌµÄKMnO4ČÜŅŗ£¬Ö¤Ć÷SO2¾ßÓŠ ŠŌ”£
£Ø3£©EÖŠ¼ÓČėµĪÓŠ·ÓĢŖµÄNaOHČÜŅŗ£¬Ö¤Ć÷SO2ŹĒ ŠŌĘųĢ唣
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźĖÄ“ØŹ”Ć¼É½ÖŠŃ§øßŅ»ĻĀѧʌʌĩ½Ģѧ֏Įæ¼ģ²ā»ÆѧŹŌĢā ĢāŠĶ£ŗŹµŃéĢā
£Ø11·Ö£©
¢ń£®¼×Ķ¬Ń§ĄūÓĆĻĀĮŠ×°ÖĆŃé֤ľĢæÓėÅØĮņĖį·“Ó¦µÄČ«²æ²śĪļ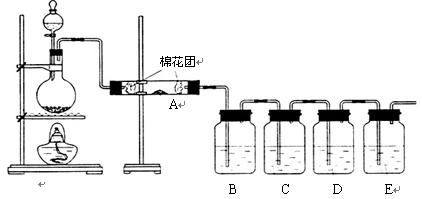
£Ø1£©Š“³öľĢæÓėÅØĮņĖį·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
£Ø2£©AÖŠ¼ÓČėµÄŹŌ¼ĮŹĒ £¬B”¢DÖŠ¼ÓČėµÄŹŌ¼Į¶¼ŹĒĘ·ŗģČÜŅŗ£¬DÖŠÕżČ·µÄŹµŃéĻÖĻóŹĒ£ŗ
£Ø3£©ŹµŃ鏱£¬CÖŠČō¼ÓČėµÄŹĒµĪÓŠµķ·ŪµÄµāĖ®£¬¹Ū²ģµ½µÄĻÖĻóŹĒ £¬
Ąė×Ó·½³ĢŹ½ĪŖ£ŗ ”£
¢ņ£®ŅŅĶ¬Ń§Ö»ÓĆB”¢C”¢D”¢E×°ÖĆŃéÖ¤SO2µÄijŠ©ŠŌÖŹ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©CÖŠ¼ÓČėµÄŹŌ¼ĮŹĒ £¬Ö¤Ć÷SO2¾ßÓŠŃõ»ÆŠŌ”£
£Ø2£©DÖŠ¼ÓČėĖįŠŌµÄKMnO4ČÜŅŗ£¬Ö¤Ć÷SO2¾ßÓŠ ŠŌ”£
£Ø3£©EÖŠ¼ÓČėµĪÓŠ·ÓĢŖµÄNaOHČÜŅŗ£¬Ö¤Ć÷SO2ŹĒ ŠŌĘųĢ唣
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźĖÄ“ØŹ”øßŅ»ĻĀѧʌʌĩ½Ģѧ֏Įæ¼ģ²ā»ÆѧŹŌĢā ĢāŠĶ£ŗŹµŃéĢā
£Ø11·Ö£©
¢ń£®¼×Ķ¬Ń§ĄūÓĆĻĀĮŠ×°ÖĆŃé֤ľĢæÓėÅØĮņĖį·“Ó¦µÄČ«²æ²śĪļ
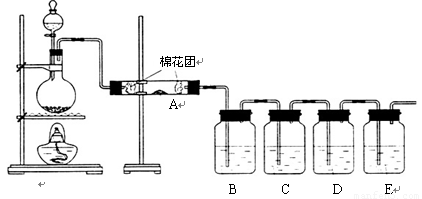
£Ø1£©Š“³öľĢæÓėÅØĮņĖį·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
£Ø2£©AÖŠ¼ÓČėµÄŹŌ¼ĮŹĒ £¬B”¢DÖŠ¼ÓČėµÄŹŌ¼Į¶¼ŹĒĘ·ŗģČÜŅŗ£¬DÖŠÕżČ·µÄŹµŃéĻÖĻóŹĒ£ŗ
£Ø3£©ŹµŃ鏱£¬CÖŠČō¼ÓČėµÄŹĒµĪÓŠµķ·ŪµÄµāĖ®£¬¹Ū²ģµ½µÄĻÖĻóŹĒ £¬
Ąė×Ó·½³ĢŹ½ĪŖ£ŗ ”£
¢ņ£®ŅŅĶ¬Ń§Ö»ÓĆB”¢C”¢D”¢E×°ÖĆŃéÖ¤SO2µÄijŠ©ŠŌÖŹ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©CÖŠ¼ÓČėµÄŹŌ¼ĮŹĒ £¬Ö¤Ć÷SO2¾ßÓŠŃõ»ÆŠŌ”£
£Ø2£©DÖŠ¼ÓČėĖįŠŌµÄKMnO4ČÜŅŗ£¬Ö¤Ć÷SO2¾ßÓŠ ŠŌ”£
£Ø3£©EÖŠ¼ÓČėµĪÓŠ·ÓĢŖµÄNaOHČÜŅŗ£¬Ö¤Ć÷SO2ŹĒ ŠŌĘųĢ唣
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 Š“³öľĢæÓėÅØĮņĖį·“Ó¦µÄ»Æѧ·½³ĢŹ½______£®
Š“³öľĢæÓėÅØĮņĖį·“Ó¦µÄ»Æѧ·½³ĢŹ½______£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com