
| A�� | 0.1mol/LCH3COOH��Һ����ˮϡ�����У���������Ũ�Ⱦ���С | |
| B�� | Ũ�Ⱦ�Ϊ0.1mol/L��NaF��CH3COONa��Һ��Ƚϣ�CH3COONa��ҺpH�� | |
| C�� | ��ӦHF+CH3COONa�TNaF+CH3COOH���Է��� | |
| D�� | NaF��Һ�м�����NaOH���壬��Һ��c��F-����� |
���� A�������ˮϡ������ֻ��c��OH-������������Ũ�ȶ���С��ˮ���ӳ��⣩��
B����ĵ���ƽ�ⳣ��Խ�����������ˮ��̶�ԽС����ͬŨ�ȵ�������ҺpHԽС��
C����ĵ���ƽ�ⳣ��Խ���������Խǿ������ǿ����ȡ�����жϣ�
D����������ǿ�������Σ�������ˮ�����Һ�ʼ��ԣ�����Һ�м���NaOH����NaFˮ�⣮
��� �⣺A�������ˮϡ�ʹٽ�������룬�������������̶�С����Һ�������̶ȣ�������Һ��c��H+����С���¶Ȳ��䣬��Һ�����ӻ��������䣬������Һ��c��OH-������������Ũ�ȶ���С��ˮ���ӳ��⣩����A����
B����ĵ���ƽ�ⳣ��Խ�����������ˮ��̶�ԽС����ͬŨ�ȵ�������ҺpHԽС�����ݵ���ƽ�ⳣ��֪������HF��CH3COOH��������ͬŨ�ȵ�NaF��CH3COONa��NaFˮ��̶�С��CH3COONa������ҺpHǰ��С�ں��ߣ���B��ȷ��
C����ĵ���ƽ�ⳣ��Խ���������Խǿ�����ݵ���ƽ�ⳣ��֪������HF��CH3COOH������ǿ����ȡ�����жϣ���ӦHF+CH3COONa�TNaF+CH3COOH���Է�������C��ȷ��
D����������ǿ�������Σ�������ˮ�����Һ�ʼ��ԣ�����Һ�м���NaOH����Һ��c��OH-�����������NaFˮ�⣬��D��ȷ��
��ѡA��
���� ���⿼��������ʵĵ��룬Ϊ��Ƶ���㣬��ȷ�������ƽ�ⳣ�������Թ�ϵ�����Ӧ���������ˮ��̶ȹ�ϵ�ǽⱾ��ؼ����״�ѡ����A��ע���ˮϡ�ʹٽ�������뵫c��H+����С������������Ŀ�ѶȲ���


 �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | P��Q��R��S��Ũ����� | B�� | P��Q��R��S���ܱ������й��� | ||
| C�� | P��Q��R��S��Ũ�Ȳ��ٱ仯 | D�� | �����ڵ�ѹǿ���ٱ仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
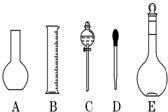 ʵ������Ҫ0.1mol/L NaOH��Һ450mL��0.5mol/L������Һ450mL��������������Һ����������ش��������⣺
ʵ������Ҫ0.1mol/L NaOH��Һ450mL��0.5mol/L������Һ450mL��������������Һ����������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
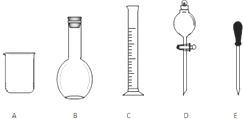 ʵ������Ҫ0.1mol/L NaOH��Һ480mL��0.5mol/L��������Һ500mL��������������Һ����������ش��������⣮
ʵ������Ҫ0.1mol/L NaOH��Һ480mL��0.5mol/L��������Һ500mL��������������Һ����������ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����һ����Ҫ�Ľ������������������о���������Ҫ����;����ͼ�Ǵ����������Ƹ����Ĺ������̣�
����һ����Ҫ�Ľ������������������о���������Ҫ����;����ͼ�Ǵ����������Ƹ����Ĺ������̣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | HCOOH | HCN | H2CO3 |
| ����ƽ�ⳣ�� �� 25�棩 | Ka=1.77��10-4 | Ka=5.0��10-10 | Ka1=4.3��10-7 Ka2=5.6��10-11 |
| A�� | ��NaCN��Һ��ͨ��������CO2���������ӷ�ӦΪ��2CN-+H2O+CO2�T2HCN+CO32- | |
| B�� | ������CN-��ˮʱ������NaOH��Һ����pH��9����ʱc��CN-����c��HCN�� | |
| C�� | �к͵��������pH��HCOOH��Һ��HCN��Һ����NaOH�����ʵ���ǰ��С�ں��� | |
| D�� | ������������ʵ���Ũ�ȵ�HCOONa��NaCN��Һ��������������ǰ�ߴ��ں��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �����£�ȡŨ�Ⱦ�Ϊ0.01mol•L-1��HA��Һ��MOH��Һ��20mL���ֱ���0.01mol•L-1NaOH��Һ��0.01mol•L-1��������к͵ζ����ζ�������pH��μ���Һ������仯��ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
�����£�ȡŨ�Ⱦ�Ϊ0.01mol•L-1��HA��Һ��MOH��Һ��20mL���ֱ���0.01mol•L-1NaOH��Һ��0.01mol•L-1��������к͵ζ����ζ�������pH��μ���Һ������仯��ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | HA��MOH��Ϊ������� | |
| B�� | ����a�У��μ���Һ��20mLʱ��c��Cl-��=c��M+��=c��OH-��=c��H+�� | |
| C�� | ����b�У��μ���Һ��20mLʱ��c��Na+����c��A-����c��OH-����c��H+�� | |
| D�� | ����b�У��μ���Һ��10mLʱ��c��A-��+c��OH-��=c��H+��+c��HA�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
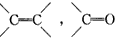 �IJ����ͶȾ�Ϊ1�����нṹ��ʽΪ
�IJ����ͶȾ�Ϊ1�����нṹ��ʽΪ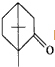 �����ʣ�ͨ��̼����ԭ�ӺͲ����Ͷȵļ��㣬ȷ�����������в����������ʵ�ͬ���칹����ǣ�������
�����ʣ�ͨ��̼����ԭ�ӺͲ����Ͷȵļ��㣬ȷ�����������в����������ʵ�ͬ���칹����ǣ�������| A�� | 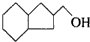 | B�� |  | C�� | 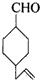 | D�� | 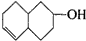 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com