
��ѧ�ܵ�ת������ʵ�����еõ��˹㷺�����á��ش��������⣺
����(1)��25�桢101kPa�£�1g������ȫȼ������CO2��Һ̬H2O���ų�55 kJ��������д����ʾ����ȼ�յ��Ȼ�ѧ����ʽ�� ��
(2)2Zn��s��+O2��g��=2ZnO��s�� ��H1=" ��702" kJ/mol
2Hg��l��+O2��g��=2HgO��s�� ��H2=" ��182" kJ/mol
�ɴ˿�֪ZnO��s��+Hg��l��= Zn��s��+HgO��s�� ��H3= ��
��3��20����30�����Eyring��Pelzer����ײ���۵Ļ����������ѧ��Ӧ�Ĺ���̬���ۣ���ѧ��Ӧ������ͨ������ײ������ɵģ������ڷ�Ӧ�ﵽ������Ĺ����о���һ������������̬����ͼ��NO2��CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ�� 
������ͼΪ������ļ����������أ�
��ش�
��1���׳���Ϊ�õ��ԭ������ͭ��װ�ã���
A�������������������� ���缫��ӦΪ����������������������
B�������������������� ���缫��ӦΪ����������������������
�������ҺΪ������������������
��2���ҳ���������������̪��Һ����ʼһ��ʱ���Fe������������������ɫ��
��3�����ײ���������12��8g�����Ҳ������ų������ڱ�״���µ����
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��4��ͬʱ���Ҳ�ʣ��Һ��Ϊ400mL�������õ���Һ�����ʵ���Ũ��Ϊ
__ ������ _____��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
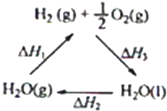 ij��ѧ�����ö������棨CeO2����̫���������½�H2O��CO2ת���H2��CO����������£�
ij��ѧ�����ö������棨CeO2����̫���������½�H2O��CO2ת���H2��CO����������£�| ̫���� |
| �� |
| 900�� |
| �� |
| A���ù�����CeO2û������ |
| B���ù���ʵ����̫������ѧ�ܵ�ת�� |
| C��ͼ�С�H1=��H2+��H3 |
| D����CO��O2���ɵļ���ȼ�ϵ�صĸ�����ӦʽΪCO+4OH--2e-=CO32-+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

ͼ1-4-7
(1)д��A��B��C��D��E�����ơ�
(2)д����Ӧ�ڵĻ�ѧ����ʽ�������л���Ӧ����һ�����͡�
(3)˵����Ӧ�ݵIJ������ճ������г���Ϊ����ķ��������������ΪʳƷ����ԭ��ͬʱ����Ҳ�ǡ���ɫ��Ⱦ���Ļ���֮һ����ԭ����ʲô?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ�ܵ�ת������ʵ�����еõ��˹㷺�����á��ش��������⣺
����(1) ��25�桢101kPa�£�1g������ȫȼ������CO2��Һ̬H2O���ų�55 kJ��������д����ʾ����ȼ�յ��Ȼ�ѧ����ʽ��
��
(2)2Zn��s��+O2��g��=2ZnO��s�� ��H1 = ��702 kJ/mol
2Hg��l��+O2��g��=2HgO��s�� ��H2= ��182 kJ/mol
�ɴ˿�֪ZnO��s��+Hg��l��= Zn��s��+HgO��s�� ��H3= ��
��3��20����30�����Eyring��Pelzer����ײ���۵Ļ����������ѧ��Ӧ�Ĺ���̬���ۣ���ѧ��Ӧ������ͨ������ײ������ɵģ������ڷ�Ӧ�ﵽ������Ĺ����о���һ������������̬����ͼ��NO2��CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��
������ͼΪ������ļ����������أ�
��ش�
��1���׳���Ϊ�õ��ԭ������ͭ��װ�ã���
A�������������������� ���缫��ӦΪ����������������������
B�������������������� ���缫��ӦΪ����������������������
�������ҺΪ������������������
��2���ҳ���������������̪��Һ����ʼһ��ʱ���Fe������������������ɫ��
��3�����ײ���������12��8g�����Ҳ������ų������ڱ�״���µ����
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��4��ͬʱ���Ҳ�ʣ��Һ��Ϊ400mL�������õ���Һ�����ʵ���Ũ��Ϊ
__ ������ _____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡΫ���и߶�������ҵ��ѧ��һ���Ծ� ���ͣ������
��ѧ�ܵ�ת������ʵ�����еõ��˹㷺�����á��ش��������⣺
����(1) ��25�桢101kPa�£� 1g������ȫȼ������CO2��Һ̬H2O���ų�55 kJ��������д����ʾ����ȼ�յ��Ȼ�ѧ����ʽ��
��
(2)2Zn��s��+O2��g��=2ZnO��s�� ��H1 = ��702 kJ/mol
2Hg��l��+O2��g��=2HgO��s�� ��H2 = ��182 kJ/mol
�ɴ˿�֪ZnO��s��+Hg��l��= Zn��s��+HgO��s�� ��H3= ��
��3��20����30�����Eyring��Pelzer����ײ���۵Ļ����������ѧ��Ӧ�Ĺ���̬���ۣ���ѧ��Ӧ������ͨ������ײ������ɵģ������ڷ�Ӧ�ﵽ������Ĺ����о���һ������������̬����ͼ��NO2��CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��

������ͼΪ������ļ����������أ�

��ش�
��1���׳���Ϊ�õ��ԭ������ͭ��װ�ã���
A�������������������� ���缫��ӦΪ����������������������
B�������������������� ���缫��ӦΪ����������������������
�������ҺΪ������������������
��2���ҳ���������������̪��Һ����ʼһ��ʱ���Fe������������������ɫ��
��3�����ײ���������12��8g�����Ҳ������ų������ڱ�״���µ����
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��4��ͬʱ���Ҳ�ʣ��Һ��Ϊ400mL�������õ���Һ�����ʵ���Ũ��Ϊ
__ ������ _____��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com