
���Ļ�������ijЩ�����а�������Ҫ�Ľ�ɫ�����������ǹ�ҵ������ǿ��֮һ��
(1)�Դ�ԭ�ϵ������ʺͻ������������Ƕȷ�����
�ܽ�����Ľ���ͭ����Ũ����ã�������ϡ����ã�д����Ӧ�Ļ�ѧ����ʽ������Ҫ���ͣ�________________��
(2)�����벻ͬŨ�ȵ����ᷴӦʱ���������ɶ��ֲ�ͬ��̬�Ļ�ԭ�����ͼ�Ǹ��ֲ�ͬ�ܶȵ�����������Ӧʱ(��ͬ�¶�)�Ļ�ԭ����ֲ�ͼ��
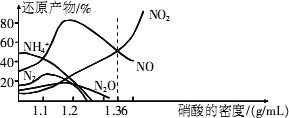
��ij�����Լ�ƿ�ı�ǩע�����ܶȣ�
1.26 g/mL������������50.0������ȡ���Լ�10 mL���1000 mL��Һ��������Һ�����ʵ���Ũ��Ϊ________���ڵ�������Һ���ܶ�Ϊ
1.36 g/mLʱ�����л�ѧ����ʽ���ܽ�ȷ�ر���Fe��������ᷴӦ����________ (�����)��a��2Fe��10HNO3![]() 2Fe(NO3)3��NO����3NO2����5H2O
2Fe(NO3)3��NO����3NO2����5H2O
b��4Fe��18HNO3![]() 4Fe(NO3)3��3NO����3NO2����9H2O
4Fe(NO3)3��3NO����3NO2����9H2O
c��2Fe��6HNO3![]() 2Fe(NO3)2��NO����NO2����3H2O
2Fe(NO3)2��NO����NO2����3H2O
(3)�����谷[C3N3(NH2)3]��һ����Ҫ���л�����ԭ�ϣ����京�����ߴ�66.7�����������̼�����ʳƷ���Ӽ���������ʳƷ����еĵ����ʺ���(�Ե�Ԫ�ص���������N����ʾ)��ijƷ���̷���N��Ϊ1.9��������100 g���̷��м���10 g�����谷����N�����Ϊԭ����________��(ȡ����)��
 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2011?�ൺģ�⣩���Ļ�������ijЩ�����а�������Ҫ�Ľ�ɫ��
��2011?�ൺģ�⣩���Ļ�������ijЩ�����а�������Ҫ�Ľ�ɫ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ���Ļ�������ijЩ�����а�������Ҫ�Ľ�ɫ��
���Ļ�������ijЩ�����а�������Ҫ�Ľ�ɫ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���ൺģ�� ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ɽ��ʡ�ൺ�и߿���ѧģ���Ծ���3�·ݣ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com