

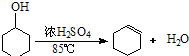
| �ܶ� ��g/cm3�� | �۵� ���棩 | �е� ���棩 | �ܽ��� | |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |
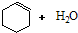
| ���� |
 ��
��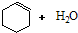
| ���� |
 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1molFeCl3��ȫˮ������1molFe��OH��3���� |
| B�����³�ѹ�£�1NA��һ�ȼ�����ӵ��������22.4L |
| C��T��ʱ��1 L pH=6��ˮ�У���10-8NA��OH- |
| D��3.4g NH3�к�N-H����ĿΪ0.2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������1 mol KAl��SO4��2����Һ�м���Ba��OH��2��Һ�������������ʱ���������ܵ����ʵ���Ϊ2.5 mol |
| B��FeCl2��Fe��OH��3����ͨ�����Ϸ�Ӧ�Ƶ� |
| C����ˮ�����c��H+��Ϊ10-13mol/L����Һ��Na+��NO3-��SO42-��I-һ���ܴ������� |
| D��������Ũ�����ữ��KMnO4��Һ��H2O2��ϣ���֤��H2O2���л�ԭ�ԣ�2MnO4-+8H++5H2O2=2Mn2++5O2��+8H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��Al2 ��SO4��3��μ���NaOH��Һ�õ�������ͼ
��Al2 ��SO4��3��μ���NaOH��Һ�õ�������ͼ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | ʵ������ | ʵ���� |
| �� | ��һ���м�AgNO3��Һ | �а�ɫ�������� |
| �� | �ڶ����м�����NaOH��Һ������ | ֻ�ռ�����״����0.896L���壬�������ɣ�ͬʱ�õ���ҺA |
| �� | ����ҺA��ͨ�����CO2�����ð�ɫ���������ˡ�ϴ�ӡ����պ���� | ��������Ϊ2.04g |
| �� | �����ݼ�����BaCl2��Һ�����ó���������ϡ����ϴ�ӡ��������� | ��������Ϊ23.3g |
| �����ӷ��� | ���ʵ���Ũ�ȣ�mol?L-1�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����CO2ͨ�����ʯ��ˮ��Һ�У�ֱ������ |
| B����NaOH��Һ��ε���AlCl3��Һ�У�ֱ������ |
| C����AlCl3��Һ����ε���ϡ���� |
| D����NaOH��Һ����ε���Fe2��SO4��3��Һֱ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1��NaOH��Һ |
| 2��ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ʹ�÷ֹ��ȼƣ����Ի���õ�ij��Һ��Ũ�� |
| B���ȼҵ�������ӽ���Ĥ���۵��ʱ����������ע�뾭�����Ƶ� NaCl��Һ����������ע��ϡ����������Һ����ȥ����ˮ�� |
| C��������ˮΪ֮��������ˮ��˵����ͬ������ˮ�ͱ���ˮ�������� |
| D��1fs�����룩=10-12s���룩 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com