

|



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
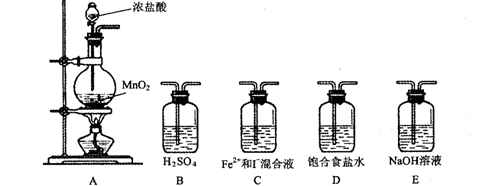
 (�����������˳���������д)
(�����������˳���������д) ��ʵ�鿪ʼ����ͨ������������ͨ���۲���Һ��ɫ�ı仯�� (�ܻ���)�жϷ�Ӧ���Ⱥ�˳��
��ʵ�鿪ʼ����ͨ������������ͨ���۲���Һ��ɫ�ı仯�� (�ܻ���)�жϷ�Ӧ���Ⱥ�˳��| ʵ�鲽�� | Ԥ����������� |
| ȡ������Ӧ����Һ����A��B��֧�Թ��У� ��A�Թ��еμ� �� ��B�Թ��еμ� �� | �� �� �����1���� �� �� �����2���� �� �� �����3���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
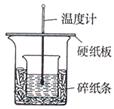
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 Ϊ��ɫ��������)������ȡ200g��Ҷ��Ʒ���յûҷۺ�������²�����
Ϊ��ɫ��������)������ȡ200g��Ҷ��Ʒ���յûҷۺ�������²�����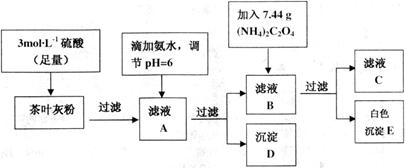
| ���� | 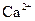 | 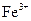 |
| ��ȫ����ʱ��pH | 13 | 4.1 |
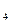 ����Һ�ζ�C��Һʱ�������ķ�ӦΪ��
����Һ�ζ�C��Һʱ�������ķ�ӦΪ��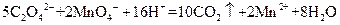 ��
��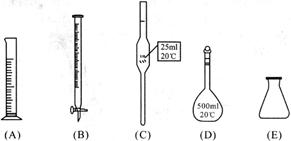
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
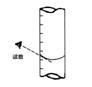
 �����2��ȷ��
�����2��ȷ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
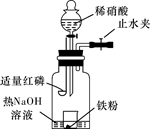
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ��� | ʵ������ | ʵ��Ŀ�� |
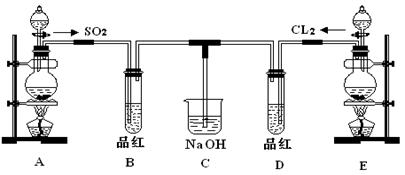
| A | ��SO2ͨ������KMnO4��Һ�� | ֤��SO2����Ư���� |
| B | ��Cl2ͨ��NaBr��Һ�У�Ȼ�����CCl4�������� | �Ƚ��������������ǿ�� |
| C | ��ͭƬ�ֱ���Ũ��ϡ���ᷴӦ | ̽��Ũ��ϡ���������Ե����ǿ�� |
| D | ��ʢ��20g���ǵ��ձ��м��뼸��ˮ��������ȡ��ټ�������Ũ���ᣬѸ�ٽ��衣 | ̽��Ũ�������ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
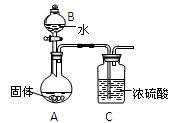
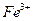 ����ܺ���
����ܺ���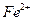 ����Ҫȷ�����е�
����Ҫȷ�����е�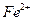 ��Ӧѡ��
��Ӧѡ��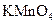 ��Һ
��Һ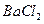 ��Һ�����ʵ�������ø������2.33g���ɴ���֪����Y��
��Һ�����ʵ�������ø������2.33g���ɴ���֪����Y��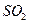 ���������Ϊ ��
���������Ϊ ��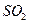 ��������Ľ����ͬѧ����Ϊ����Y�л����ܺ������������岢�����²��룺
��������Ľ����ͬѧ����Ϊ����Y�л����ܺ������������岢�����²��룺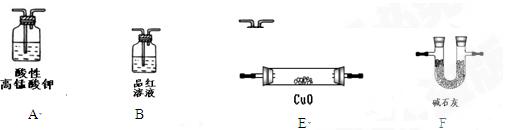
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com