



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������̪�Ժ�ɫ����Һ��Na+��Mg2+��NO3����HSO3�� |
| B��0.1 mol��L��1 Fe(NO3)2��Һ��H+��Al3+��SO42����Cl�� |
| C��0.1 mol��L��1��ˮ��Һ��K+��Na+��NO3����AlO2�� |
| D����ˮ�������c(H+)=10��11mol��L��1����Һ��Ca2+��NH4+��Cl����HCO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ������ | 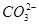 �� ��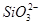 �� ��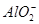 ��Cl�� ��Cl�� |
| ������ | Al3����Cu2����Mg2���� ��Na�� ��Na�� |
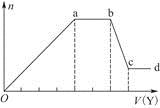
 Sn(OH)2
Sn(OH)2 Sn2����2OH����
Sn2����2OH�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
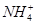 ��Cl����
��Cl����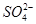 ��
��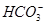 ��
��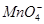 �еļ��֡�Ϊȷ����ɷ֣�������ʵ�飺��ȡ������Һ������������Na2O2���壬������ɫ��ζ������Ͱ�ɫ�������ټ���������NaOH��Һ���ɫ���������ܽ⣻����ȡ������Һ������HNO3�ữ��Ba(NO3)2��Һ���а�ɫ���������������ƶ���ȷ����
�еļ��֡�Ϊȷ����ɷ֣�������ʵ�飺��ȡ������Һ������������Na2O2���壬������ɫ��ζ������Ͱ�ɫ�������ټ���������NaOH��Һ���ɫ���������ܽ⣻����ȡ������Һ������HNO3�ữ��Ba(NO3)2��Һ���а�ɫ���������������ƶ���ȷ����A���϶���Al3����Mg2����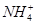 ��Cl�� ��Cl�� |
B���϶���Al3����Mg2����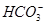 |
C���϶���K����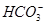 �� ��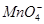 |
D���϶���Al3����Mg2����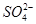 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ˮ�����ӻ�����KWֻ���¶��йأ�������ᡢ���һ����Ӱ��ˮ�ĵ���̶� |
| B��Ksp���������ܵ���ʵ����ʺ��¶��йأ�������Һ��������ӵ�Ũ���й� |
C�������£���0.10 mol��L��1��NH3��H2O��Һ�м�������NH4Cl���壬��ʹ��Һ��pH��С��c(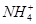 )/c(NH3��H2O)��ֵ���� )/c(NH3��H2O)��ֵ���� |
| D�������£�CH3COOH��KW��1.7��10��5��NH3��H2O��Kb��1.7��10��5��CH3COOH��Һ�е�c(H��)��NH3��H2O�е�c(OH��)��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ij������Һ��pH>7���������һ���Ǽ��ǿ�������� |
| B��pH��6.5��ţ����c(H��)��pH��4.5��H2SO4��Һ��c(H��)��100�� |
| C��pH��3�Ĵ�����pH��11��NaOH��Һ�������Ϻ���Һ�У� c(CH3COO��)>c(Na��)>c(H��)>c(OH��) |
| D��AgCl�ڵ�Ũ�ȵ�CaCl2��Һ��NaCl��Һ�е��ܽ����ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ˮ�еμ�ŨH2SO4��Kw���� |
B��CH3COOH��Һ��ˮϡ�ͺ���Һ��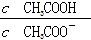 ��ֵ��С ��ֵ��С |
C��Na2CO3��Һ�м�������Ca(OH)2���壬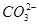 ˮ��̶ȼ�С����Һ��pH��С ˮ��̶ȼ�С����Һ��pH��С |
| D��NaCl��Һ��CH3COONH4��Һ�������ԣ�����Һ��ˮ�ĵ���̶���ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��25��ʱ NH4Cl ��Һ�� KW���� 100��ʱ NaCl ��Һ�� KW |
B��SO2ͨ���ˮ�У���Ӧ�����ӷ���ʽΪ SO2��I2��2H2O=SO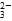 ��2I����4H�� ��2I����4H�� |
C�����������ܲ��� H2����Һ�У����ܴ��ڴ����� Na����Ba2����AlO ��NO ��NO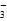 |
| D��100��ʱ���� pH ��2 �������� pH ��12 �� NaOH ��Һ�������ϣ���Һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��c(H+)=1��10-14mol/L����Һ��K����Cu2����I����SO42�� |
| B��ˮ�������c(H+)=1��10-14mol/L����Һ��K+��Na����AlO2����S2O32�� |
| C������Al��Ӧ����H2����Һ��NH4����Ca2+��NO3����I�� |
| D������K3[Fe(CN)6]������ɫ��������Һ��H����Na����SO42����CrO42�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com