
���� ��1����������һ�����ʵ���Ũ����Һ��һ�㲽��ѡ����Ҫ������
��2��������Һ���ѡ��250mL����ƿ������m=CVM������Ҫ���ʵ�������
��3���������������׳�������ʽ��
��4���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=$\frac{n}{V}$�жϣ�
��� �⣺��1������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ���������������ƽ�������룩���ձ�����������250 mL����ƿ����ͷ�ιܣ���ȱ�ٵ�������250 mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��250 mL����ƿ����ͷ�ιܣ�
��2���ù����ռ�����0.4mol•L-1��NaOH��Һ240mL��Ӧѡ��250mL����ƿ����Ҫ���ʵ�����m=0.4mol/L��
40g/mol��0.25L=4.0g��
�ʴ�Ϊ��4.0��
��3�������ռ�Ķ���ҪѸ�٣�ԭ���ǣ���ֹ�ռ�����ڿ�������ˮ���⣻
�ʴ�Ϊ����ֹ�ռ�����ڿ�������ˮ���⣻
��4��A��NaOH���ձ����ܽ������ת�Ƶ�����ƿ�����������ܽ���ȣ���Һ���ݺ���ȴ�����£�Һ���½�����Һ���ƫС����ҺŨ��ƫ�ߣ���Aѡ��
B������ʱ���Ӷ�����������Һ���ƫС����ҺŨ��ƫ�ߣ���Bѡ��
C�����ݡ�ҡ�ȡ����ã�����Һ����ڿ��ߣ��ټ�ˮ���̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���C��ѡ��
D������ƿû�и��T����������Һ�������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣬��D��ѡ��
E���ܽ��ռ���ձ�δϴ�ӣ����²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���E��ѡ��
��ѡ��AB��
���� ���⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ�������Ŀ�Ѷ��еȣ�ע����������һ�����ʵ���Ũ�ȵ���Һ���������ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����������ѵ�������������ע����ȷ�������ķ�����


 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Al3+��NH4+��Cl-��CO32- | B�� | Na+��CH3COO-��K+��HCO3- | ||
| C�� | Fe2+��Cl-��Na+��NO3- | D�� | K+��I-��Cl-��Rb+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������Һ��ͯ����6.4g | B�� | ������������6.4g | ||
| C�� | �������ӵ�����С��6.4g | D�� | ��Һ��Cu2+Ũ����ȫ���ֲ��䣮 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ȼ�գ����ɶ�����̼��ˮ | |
| B�� | ��Ȳ�����ӳɷ�Ӧ�����鲻�ܷ����ӳɷ�Ӧ | |
| C�� | ���ܱ��������������ʹ���Ը��������Һ��ɫ | |
| D�� | ��ͬ���ʵ������������Ȳ����ȫȼ��ʱ����CO2����ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ʵ���ǽ��л�ѧ�о�����Ҫ�ֶ�֮һ����ش��������⣺
ʵ���ǽ��л�ѧ�о�����Ҫ�ֶ�֮һ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
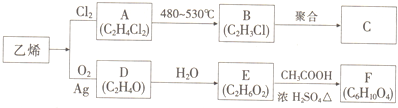

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2O2 Al2��SO4��3 MgCl2 KCl | |
| B�� | BaCl2 Na2SO4 ��NH4��2SO4 KOH | |
| C�� | AgNO3 NaCl KCl CuCl2 | |
| D�� | Fe2��SO4��3 NaOH ϡ���� NH4Cl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

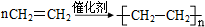 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com