
����������(Na2S2O3)�dz��õ�ʳƷ��������֮һ���ڿ����б��������ɵIJ���ΪNa2SO4.ijС���������ʵ�飺
ʵ��һ ���������Ƶ���ȡ
������ͼװ��(ʵ��ǰ�ѳ���װ���ڵĿ���)��ȡNa2S2O5��װ��II����Na2S2O5���������������ķ�ӦΪ��Na2SO3+SO2=Na2S2O5
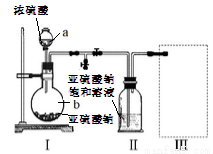
��1������a��b�����Ʒֱ���_________��__________��
��2��װ��I�в�������Ļ�ѧ����ʽΪ__________��
��3��Ҫ��װ��II�л���������ľ��壬�ɲ��õķ��뷽����__________���÷����õ��IJ����������ձ���©������___________��
��4��װ��III���ڴ���β������ѡ�õ������װ��(�г���������ȥ)Ϊ_________(�����)��
ʵ��� ���������Ƶ�����
Na2S2O5����ˮ������NaHSO3
��5��NaHSO3��Һ��HSO3-�ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����_________(�����)��
A���ⶨ��Һ��pH
B������Ba(OH)2��Һ
C����������
D������Ʒ����Һ
E.����ɫʯ����ֽ���
��6������Na2S2O5�����ڿ������ѱ�������ʵ�鷽����________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�찲��ʡ������ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£���ͬpH���������ƺʹ�������Һ�ֱ��ˮϡ�ͣ�ƽ��ʱpH����Һ����仯����������ͼ��ʾ��������������ȷ����
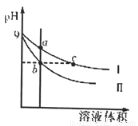
A��b��c������Һ�ĵ���������ͬ
B��a��b��c������Һ��ˮ�ĵ���̶�a��c��b
C��c����Һ��c(H+)=c(OH��)+c(CH3COOH)
D���õ�Ũ�ȵ�����ֱ���������b��c����Һǡ����ȫ��Ӧ�������������Vb=Vc
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ�߶��Ͽ�ѧ����ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й����ʽṹ��˵����ȷ����
A��78g Na2O2�������������������Ӹ�����Ϊ4NA
B��HBr�ĵ���ʽΪ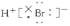
C��3.4g�������0.6NA��N����H��
D��PCl3��H2O����������ԭ�ӵ�����㶼�ﵽ8�����ȶ��ṹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������8�¿�ѧ����ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ʵ�ת���ڸ�����������ʵ�ֵ���( )
��
��
��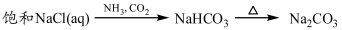
��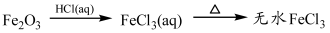
��
A. �ڢܢ� B. �ڢۢ� C.�٢ۢ� D. �٢ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������8�¿�ѧ����ѧ�Ծ��������棩 ���ͣ�ѡ����
�ҹ���������ݸ�Ŀʰ�š��м���ҩ��dz�ˮ����д���������Բ���ƿ��������ڣ���ʹй������ҩ���������������ң������ԣ����в������ᡣ����������֮�������ã����������������ġ��dz�ˮ����ָ( )
A������ B����ˮ C���� D��±ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������8���¿����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ͼʾʵ����ȷ����( )
A��ʵ�����Ʊ�����
B������п�����ᷴӦ������
C������������ʴ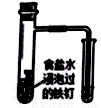
D��ʵ����������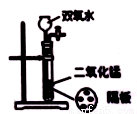
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ������ѧ���ܿ���8.28����ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪NO2��NaOH��Һ��ӦΪ��3NO2+2NaOH=2NaNO3+NO+H2O��NO��NO2��һ����NaOH��Һ���ã�NO+NO2+2NaOH=2NaNO2+H2O����ʢa molNO��b molNO2��c molO2���ܱ������У�����V LijŨ�ȵ��ռ���Һ���ܱ�������ѹǿ����Ϊ�㡣��NaOH��Һ�����ʵ���Ũ�ȣ�mol/L��Ϊ�� ��
A�� B��
B�� C��
C�� D��
D��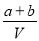
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ������ѧ���ܿ���8.28����ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӷ���ʽ��ȷ���ǣ���
A����Ba(OH)2��Һ��ϡH2SO4��Һ�����кͷ�Ӧ��Ba2++OH-+H++SO42-=BaSO4��+H2O
B��H2Sͨ��FeCl3��Һ�У�2Fe3++S2-=2Fe2++S��
C��̼��������Һ������������Һ��Ӧ��H++HCO3-=CO2��+H2O
D����Ư����Һ��ͨ��SO2: Ca2++2ClO-+H2O+SO2=CaSO3��+2HClO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ��һ����ѧ���Ի�ѧ���������棩 ���ͣ�ѡ����
�������ʵ���;�������������йص���
A���ð״�ϡ����ϴȥˮ������ˮƿ���ϵ�ˮ��
B����ʯī���ɵ�صĵ缫
C������ͷʱ���ڷ��ͺ�������м��������Ĵ��ʹ�������ͷ���ɶ��
D������ʯ����������Ȱ�װʳƷ�ĸ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com