
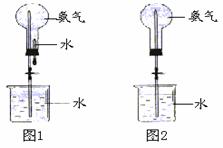
 ��ȡ�����������Ȫʵ�飨ͼ�мг�װ�þ�����ȥ����
��ȡ�����������Ȫʵ�飨ͼ�мг�װ�þ�����ȥ����
(1)д��ʵ������ȡ�����Ļ�ѧ����ʽ��
(2)�ռ�����Ӧʹ�� ����Ҫ�õ�����İ�����ѡ�� �������
(3)��μ�����ƿ�ڵİ����Ѿ��ռ�����
��
��ͼ1װ�ý�����Ȫʵ�飬�ϲ���ƿ��װ������İ���������ˮ����IJ����ǣ���ѹ��ͷ�ιܣ���ֹˮ�С���ʵ���ԭ���� ��
һ������Ȫʵ��ʱ��ˮ�������ܽ���ƿ����������Ҫԭ����
���ֻ�ṩ��ͼ2��װ�ã���˵��������Ȫ�ķ�����
��
 С�����ϵ�д�
С�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
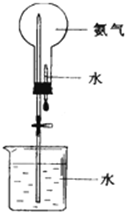 ��ȡ�����������Ȫʵ�飨ͼ�мг�װ�þ�����ȥ��
��ȡ�����������Ȫʵ�飨ͼ�мг�װ�þ�����ȥ��
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ѹǿ����������ʵ��ͻ�ѧʵ��������ҪӰ�죬��ȡ�����������Ȫʵ�飮
����ѹǿ����������ʵ��ͻ�ѧʵ��������ҪӰ�죬��ȡ�����������Ȫʵ�飮
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
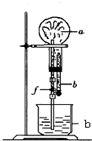 ��ȡ�����������Ȫʵ�飺
��ȡ�����������Ȫʵ�飺
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
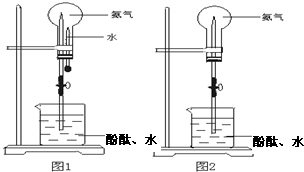
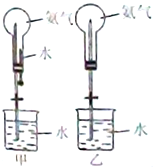 ��ȡ�����������Ȫʵ�飨ͼ�мг�װ�þ�����ȥ��
��ȡ�����������Ȫʵ�飨ͼ�мг�װ�þ�����ȥ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com