
£Ø±¾Ģā16·Ö£©
(1)ij»ÆŗĻĪļ½į¹¹¼ņŹ½ČēĶ¼£¬Ēėøł¾ŻĖłŹ¾µÄ»ÆŗĻĪļ»Ų“šĪŹĢā£ŗ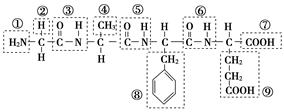
øĆ»ÆŗĻĪļÖŠ£¬¹ŁÄÜĶÅ¢ŁµÄĆū³ĘŹĒ________£»¹ŁÄÜĶŢߵÄĆū³ĘŹĒ________£»øĆ»ÆŗĻĪļŹĒÓÉ________øö°±»łĖį·Ö×ÓĶŃĖ®ŠĪ³ÉµÄ£»Š“³öøĆ»ÆŗĻĪļĖ®½āÉś³ÉµÄ°±»łĖįµÄ½į¹¹¼ņŹ½(ČĪŠ“Ņ»ÖÖ)£ŗ________£»²¢Š“³ö“Ė°±»łĖįÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ_________________
(2)ijӊ»śĪļŗ¬ÓŠC”¢N”¢H”¢OĖÄÖÖŌŖĖŲ£¬ĻĀĶ¼ĪŖøĆÓŠ»śĪļµÄĒņ¹÷Ä£ŠĶ”£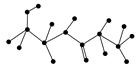
¢ŁøĆÓŠ»śĪļµÄ»ÆѧŹ½______________________________”£
½į¹¹¼ņŹ½_______________________________________________ _______”£
_______ӣ
¢ŚøĆÓŠ»śĪļæÉÄÜ·¢ÉśµÄ»Æѧ·“Ó¦ÓŠ(Ģī±ąŗÅ)________”£
a£®Ė®½ā””b£®¼Ó¾Ūc£®Č”“ś d£®ĻūČ„e£®õ„»Æ
¢ŪøĆÓŠ»śĪļ·¢ÉśĖ®½ā·“Ó¦µÄ»Æѧ·½³ĢŹ½__________________________________”£
 »„¶ÆÓ¢ÓļĻµĮŠ“š°ø
»„¶ÆÓ¢ÓļĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø±¾Ģā16·Ö£©ĮņĖį±µŹĒĪØŅ»µÄĪŽ¶¾µÄ±µŃĪ£¬¹¤ŅµÉĻŅŌĮņĖį±µµČĪŖŌĮĻĶعżĻĀĮŠĮ÷³Ģ·“Ó¦æÉŅŌÖʱøŠæ±µ°×£ØBaSO4+ZnS£©ŗĶ¹żŃõ»ÆĒā”££Øš©·ÆĪŖZnSO4•7H2O£©
£Ø1£©ÉĻŹöĮ÷³ĢÖŠ¹²ÓŠ7øö»Æѧ·“Ó¦£¬ĘäÖŠÓŠ____________øöŹōÓŚŃõ»Æ»¹Ō·“Ó¦”£
£Ø2£©Š“³ö¹żŃõ»ÆĒāŗĶĪļÖŹCµÄµē×ÓŹ½£ŗ____________________£¬_______________”£
£Ø3£©Š“³öF”¢GµÄ»ÆѧŹ½£ŗ F_____________”¢G_________________”£
£Ø4£©Š“³öĻĀĮŠ»Æѧ·“Ó¦·½³ĢŹ½£ŗ
·“Ó¦¢Ū__________________________________________________________”£
·“Ó¦¢ß____________________________________________________”£
£Ø5£©Č”Šæ±µ°×¹ĢĢå16.5gČÜÓŚ100mL 1mol/LµÄH2SO4 ČÜŅŗÖŠ£¬·Å³öH2S ĘųĢå1008mL£ØŅŃÕŪĖć³É±ź×¼×“æö£©
¢Ł²»¼ĘČÜŅŗĢå»ż±ä»Æ£¬ĖłµĆČÜŅŗÖŠĒāĮņĖįµÄĪļÖŹµÄĮæÅضČĪŖ________mol/L
¢Ś¼ÓČČĒż¾”ČÜŅŗÖŠH2S ŗó£¬ĪŖŹ¹ŠæĄė×ÓøÕŗĆĶźČ«³Įµķ£¬Ó¦¼ÓČė 1 mol/LµÄNaOHČÜŅŗ_____mL
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø±¾Ģā16·Ö£©
(1)ij»ÆŗĻĪļ½į¹¹¼ņŹ½ČēĶ¼£¬Ēėøł¾ŻĖłŹ¾µÄ»ÆŗĻĪļ»Ų“šĪŹĢā£ŗ
øĆ»ÆŗĻĪļÖŠ£¬¹ŁÄÜĶÅ¢ŁµÄĆū³ĘŹĒ________£»¹ŁÄÜĶŢߵÄĆū³ĘŹĒ________£»øĆ»ÆŗĻĪļŹĒÓÉ________øö°±»łĖį·Ö×ÓĶŃĖ®ŠĪ³ÉµÄ£»Š“³öøĆ»ÆŗĻĪļĖ®½āÉś³ÉµÄ°±»łĖįµÄ½į¹¹¼ņŹ½(ČĪŠ“Ņ»ÖÖ)£ŗ________£»²¢Š“³ö“Ė°±»łĖįÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ_________________
(2)ijӊ»śĪļŗ¬ÓŠC”¢N”¢H”¢OĖÄÖÖŌŖĖŲ£¬ĻĀĶ¼ĪŖøĆÓŠ»śĪļµÄĒņ¹÷Ä£ŠĶ”£
¢ŁøĆÓŠ»śĪļµÄ»ÆѧŹ½______________________________”£
½į¹¹¼ņŹ½______________________________________________________”£
¢ŚøĆÓŠ»śĪļæÉÄÜ·¢ÉśµÄ»Æѧ·“Ó¦ÓŠ(Ģī±ąŗÅ)________”£
a£®Ė®½ā””b£®¼Ó¾Ūc£®Č”“ś d£®ĻūČ„e£®õ„»Æ
¢ŪøĆÓŠ»śĪļ·¢ÉśĖ®½ā·“Ó¦µÄ»Æѧ·½³ĢŹ½__________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ¹ć¶«Ź”ĀŽ¶ØŹŠø߶žĻĀѧʌʌ֊֏¼ģĄķ×Ū»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
±¾Ģā16·Ö£¬¢ŁĻĀĮŠĮ½·łĘ×Ķ¼ŹĒ½į¹¹¼ņŹ½ĪŖCH3CH2CH2OHŗĶCH3CH(OH)CH3µÄĮ½ÖÖÓŠ»ś»ÆŗĻĪļµÄ1HŗĖ“Ź²ÕńĘ×Ķ¼”£ĒėÅŠ¶ĻÄÄŅ»·łŹĒCH3CH(OH)CH3µÄ1H”ŖNMRĘ×Ķ¼£¬²¢ĖµĆ÷ĄķÓÉ”££Ø6·Ö£©
¢Ś”¢0.2 molÓŠ»śĪļŗĶ0.4 mol O2ŌŚĆܱÕČŻĘ÷ÖŠČ¼ÉÕŗóµÄ²śĪļĪŖCO2”¢COŗĶH2O£Øg£©”£Č¼ÉÕŗóµÄÕāŠ©²śĪļ¾¹żÅØH2SO4ŗó£¬ÖŹĮæŌö¼Ó10.8 g£»ŌŁĶعż×ĘČȵÄCuO³ä·Ö·“Ó¦ŗ󣬹ĢĢåÖŹĮæ¼õĒį3.2 g£¬×īŗóĘųĢåŌŁĶعż¼īŹÆ»Ņ±»ĶźČ«ĪüŹÕ£¬ÖŹĮæŌö¼Ó17.6 g”££ØæÉÄÜÓƵ½µÄĻą¶ŌŌ×ÓÖŹĮæH-1£¬C-12£¬O-16£¬Cu-64£©
£Ø1£©ĶʶĻøĆÓŠ»śĪļµÄ·Ö×ÓŹ½”££Ø6·Ö£©
£Ø2£©Čō0.2 moløĆÓŠ»śĪļŌŚÓė¹żĮæµÄ½šŹōÄĘĶźČ«·“Ó¦ŗó·Å³ö4.48 L H2£Ø±ź×¼×“æö£©£¬ŹŌČ·¶ØÓŠ»śĪļµÄ½į¹¹¼ņŹ½”££Ø4·Ö£©?
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģÕć½Ź”ø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»Æѧ£ØĄķ£©ŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø±¾Ģā16·Ö£©
(1)ij»ÆŗĻĪļ½į¹¹¼ņŹ½ČēĶ¼£¬Ēėøł¾ŻĖłŹ¾µÄ»ÆŗĻĪļ»Ų“šĪŹĢā£ŗ
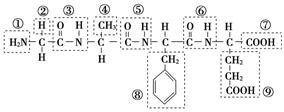
øĆ»ÆŗĻĪļÖŠ£¬¹ŁÄÜĶÅ¢ŁµÄĆū³ĘŹĒ________£»¹ŁÄÜĶŢߵÄĆū³ĘŹĒ________£»øĆ»ÆŗĻĪļŹĒÓÉ________øö°±»łĖį·Ö×ÓĶŃĖ®ŠĪ³ÉµÄ£»Š“³öøĆ»ÆŗĻĪļĖ®½āÉś³ÉµÄ°±»łĖįµÄ½į¹¹¼ņŹ½(ČĪŠ“Ņ»ÖÖ)£ŗ________£»²¢Š“³ö“Ė°±»łĖįÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ_________________
(2)ijӊ»śĪļŗ¬ÓŠC”¢N”¢H”¢OĖÄÖÖŌŖĖŲ£¬ĻĀĶ¼ĪŖøĆÓŠ»śĪļµÄĒņ¹÷Ä£ŠĶ”£
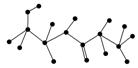
¢ŁøĆÓŠ»śĪļµÄ»ÆѧŹ½______________________________”£
½į¹¹¼ņŹ½______________________________________________________”£
¢ŚøĆÓŠ»śĪļæÉÄÜ·¢ÉśµÄ»Æѧ·“Ó¦ÓŠ(Ģī±ąŗÅ)________”£
a£®Ė®½ā””b£®¼Ó¾Ūc£®Č”“ś d£®ĻūČ„e£®õ„»Æ
¢ŪøĆÓŠ»śĪļ·¢ÉśĖ®½ā·“Ó¦µÄ»Æѧ·½³ĢŹ½__________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com