
25 ЎжЈ¬101 k PaКұЈ¬ЗҝЛбУлЗҝјоөДПЎИЬТә·ўЙъЦРәН·ҙУҰЙъіЙ1molЛ®КұЛщ·ЕіцөДИИБҝФјОӘ57.3 kJ/molЈ»РБНйөДИјЙХИИОӘ5518 kJ/molЎЈПВБРИИ»ҜС§·ҪіМКҪКйРҙХэИ·өДКЗЈЁ Ј©
AЈ®CH3COOH(aq)+NaOH(aq)=== CH3COONa(aq)+H2O(l) ![]() H=
H=![]() 57.3kJ/mol
57.3kJ/mol
BЈ®KOH(aq)+![]() H2SO4(aq)=
H2SO4(aq)= ![]() K2SO4(aq)+H2O(l)
K2SO4(aq)+H2O(l) ![]() H=
H=![]() 57.3kJ/mol
57.3kJ/mol
CЈ®2C8H18(g)+25O2 (g)=16CO2 (g)+18H2O(1) ![]() H=
H=![]() 11036 kJ/mol
11036 kJ/mol
DЈ®C8H18(l)+ ![]() O2 (g)=8CO2 (g)+ 9H2O(g)
O2 (g)=8CO2 (g)+ 9H2O(g) ![]() H=
H=![]() 5518 kJ/mol
5518 kJ/mol
 ЦРҝјҪв¶Бҝјөгҫ«Б·ПөБРҙр°ё
ЦРҝјҪв¶Бҝјөгҫ«Б·ПөБРҙр°ё
| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2012ҪмәУДПКЎҪ№ЧчКРёЯИэөЪТ»ҙОЦКБҝјмІвАнЧЫ»ҜС§Іҝ·ЦЈЁҙшҪвОцЈ© МвРНЈәМоҝХМв
(15·Ц)ЦРС§»ҜС§іЈјыІҝ·ЦФӘЛШФӯЧУҪб№№ј°РФЦКИзПВұнЛщКҫЈә
| РтәЕ | ФӘЛШ | Ҫб№№ј°РФЦК |
| ўЩ | A | AөҘЦККЗЙъ»оЦРіЈјыҪрКфЈ¬ЛьУРБҪЦЦВИ»ҜОпЈ¬Па¶Ф·ЦЧУЦКБҝПаІо35.5 |
| ўЪ | B | BФӯЧУKЎўLЎўMІгөзЧУКэЦ®ұИКЗ1:4:1 |
| ўЫ | C | CКЗ»оЖГ·ЗҪрКфФӘЛШЈ¬ЖдөҘЦКіЈОВПВіКЖшМ¬ө«»ҜС§РФЦКОИ¶Ё |
| ўЬ | D | DөҘЦКұ»УюОӘЎ°РЕПўёпГьөДҙЯ»ҜјБЎұЈ¬КЗіЈУГөД°лөјМеІДБП[АҙФҙ:Z*xx*k.Com] |
| ўЭ | E | НЁіЈЗйҝцПВЈ¬EГ»УРХэ»ҜәПјЫЈ¬AЎўCЎўF¶јДЬУлEРОіЙ¶юЦЦ»т¶юЦЦТФЙП»ҜәПОп |
| ўЮ | F | FФЪЦЬЖЪұнЦРҝЙТФЕЕФЪўсAЧеЈ¬ТІУРИЛМбіцЕЕФЪўчAЧе |
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2014ҪмХгҪӯКЎёЯ¶юЙПС§ЖЪЖЪД©ҝјКФ»ҜС§КФҫнЈЁҪвОц°жЈ© МвРНЈәСЎФсМв
25 ЎжЈ¬101 k PaКұЈ¬ЗҝЛбУлЗҝјоөДПЎИЬТә·ўЙъЦРәН·ҙУҰөДЦРәНИИОӘ-57.3 kJ/molЈ¬РБНйөДИјЙХИИОӘ-5518 kJ/molЎЈПВБРИИ»ҜС§·ҪіМКҪКйРҙХэИ·өДКЗЈЁ Ј©
AЈ®2H+(aq)
+ (aq)+
(aq)+ (aq)+2
(aq)+2 (aq) = BaSO4(s)+2H
(aq) = BaSO4(s)+2H O(l);
O(l);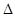 H =
H = 57.3 kJ/mol
57.3 kJ/mol
BЈ®KOH(aq)+ H
H SO4(aq) =
SO4(aq) =  K
K SO4(aq)+H
SO4(aq)+H O(l);
O(l);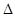 H=
H= 57.3kJ/mol
57.3kJ/mol
CЈ®C8H18(l)+
 O
O (g)=8CO
(g)=8CO (g)+ 9H
(g)+ 9H O(g);
O(g);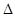 H=
H= 5518 kJ/mol
5518 kJ/mol
DЈ®2C8H18(g)+25O (g)=16CO
(g)=16CO (g)+18H
(g)+18H O(1);
O(1);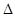 H=
H= 5518 kJ/mol
5518 kJ/mol
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2013Ҫм°І»ХКЎёЯИэөЪТ»ҙОНіҝј»ҜС§КФҫнЈЁҪвОц°жЈ© МвРНЈәСЎФсМв
25 ЎжЈ¬101 k PaКұЈ¬ЗҝЛбУлЗҝјоөДПЎИЬТә·ўЙъЦРәН·ҙУҰөДЦРәНИИОӘ57.3 kJ/molЈ¬РБНйөДИјЙХИИОӘ5518 kJ/molЎЈПВБРИИ»ҜС§·ҪіМКҪКйРҙХэИ·өДКЗ ( )
AЈ®2H+(aq)+SO42Јӯ(aq)+Ba2Ј«(aq)+2OH-(aq)=BaSO4(s)+2H2O(1)
ЎчH= 57.3 kJ/mol
57.3 kJ/mol
BЈ®KOH(aq)+ H2 SO4(aq)=
H2 SO4(aq)= K2SO4(aq)+H2O(l) ЎчH=
K2SO4(aq)+H2O(l) ЎчH= 57.3kJ/mol
57.3kJ/mol
CЈ®C8H18(I)+ O2(g)=8CO2(g)+9H2O ЎчH=
O2(g)=8CO2(g)+9H2O ЎчH= 5518 kJ/mol
5518 kJ/mol
DЈ®2C8H18(g)+25O2(g)=16CO2(g)+18H2O(1)
ЎчH= 5518 kJ/mol
5518 kJ/mol
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2013Ҫм№г¶«КЎёЯ¶юЙПС§ЖЪЖЪЦРҝјКФ»ҜС§КФҫн МвРНЈәСЎФсМв
25ЎжЈ¬101 k PaКұЈ¬ЗҝЛбУлЗҝјоөДПЎИЬТә·ўЙъЦРәН·ҙУҰөДЦРәНИИОӘ57.3 kJ/molЈ¬РБНйөДИјЙХИИОӘ5518 kJ/molЎЈПВБРИИ»ҜС§·ҪіМКҪКйРҙХэИ·өДКЗЈЁ Ј©
AЈ®2H+(aq) +SO42Јӯ(aq)+Ba2+(aq)+2OHЈӯ(aq)=BaSO4(s)+2H2O(1)Ј»ҰӨH=Јӯ57.3 kJ/mol
BЈ®KOH(aq)+ H2 SO4(aq)=K2SO4(aq)+H2O(l)Ј»ҰӨH=Јӯ57.3kJ/mol
CЈ®2C8H18(g)+25O2(g)=16CO2(g)+18H2O(1)Ј»ҰӨH=Јӯ5518 kJ/mol
DЈ®C8H18(l)+ 25/2 O2(g)=8CO2(g)+ 9H2O(1)Ј»ҰӨH=Јӯ5518 kJ/mol
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2009ёЯҝјХжМв»гұа-СхЧеФӘЛШЈ¬»·ҫіұЈ»Ө МвРНЈәСЎФсМв
25 ЎжЈ¬101 k PaКұЈ¬ЗҝЛбУлЗҝјоөДПЎИЬТә·ўЙъЦРәН·ҙУҰөДЦРәНИИОӘ57.3 kJ/molЈ¬РБНйөДИјЙХИИОӘ5518 kJ/molЎЈПВБРИИ»ҜС§·ҪіМКҪКйРҙХэИ·өДКЗ
A.2H+(aq) + (aq)+
(aq)+ (aq)+2OH
(aq)+2OH (aq)=BaSO4(s)+2H
(aq)=BaSO4(s)+2H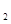 O(1);
O(1); H=
H= 57.3
kJ/mol
57.3
kJ/mol
 B.KOH(aq)+
B.KOH(aq)+ H
H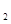 SO4(aq)=
SO4(aq)=  K
K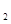 SO4(aq)+H
SO4(aq)+H O(I);
O(I);  H=
H= 57.3kJ/mol
57.3kJ/mol
C.C8H18(I)+  O
O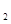 (g)=8CO
(g)=8CO (g)+ 9H
(g)+ 9H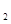 O;
O;  H=
H= 5518 kJ/mol
5518 kJ/mol
 D.2C8H18(g)+25O
D.2C8H18(g)+25O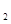 (g)=16CO
(g)=16CO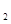 (g)+18H
(g)+18H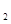 O(1);
O(1);  H=
H= 5518 kJ/mol
5518 kJ/mol
Ійҝҙҙр°ёәНҪвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com