

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
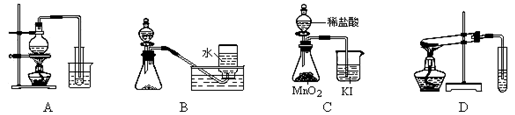
| A����ȡ��������ˮ | B����ͭ��Ũ������ȡ����NO2 |
| C���Ƚ�MnO2��Cl2��I2�������� | D��̽��NaHCO3�����ȶ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ����д���������ĸ���ϵʽ�������жϣ�����ȷ�Ĺ�ϵʽ�����
����д���������ĸ���ϵʽ�������жϣ�����ȷ�Ĺ�ϵʽ����� �����ڴ���ĺ���д����ȷ�Ĺ�ϵʽ��
�����ڴ���ĺ���д����ȷ�Ĺ�ϵʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����KI������Һ��ͨ��Cl2����Һ������˵������������۷�����ɫ��Ӧ |
| B��Ũ�����ڹ��������±��,˵��Ũ����ȶ�,����ɫ����������������Ũ���� |
| C����ij��Һ�м���HNO3�ữ��BaCl2��Һ�а�ɫ��������,˵����Һ�к���SO42- |
| D����ͭƬ����Ũ������������ʵ������˵��ͭ�����Ũ�����з����ۻ� |
�鿴�𰸺ͽ���>>
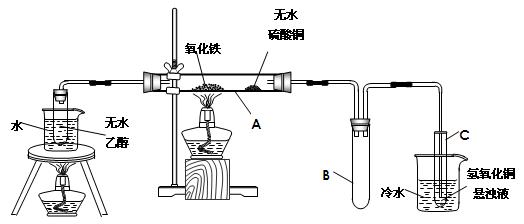
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
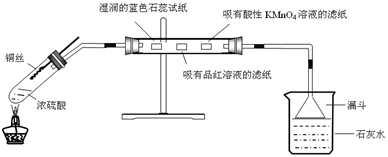
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
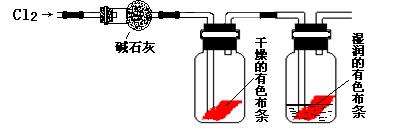
 ��
���鿴�𰸺ͽ���>>
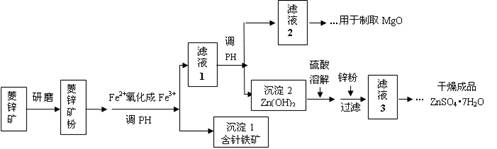
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 2HCrO
2HCrO Cr2O��H2O���ɴ��жϸõζ����˵�pHֵ��Χ��������������(�����)
Cr2O��H2O���ɴ��жϸõζ����˵�pHֵ��Χ��������������(�����)�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����������У�Ӧʹ������е�ˮδ��ȫ����ʱ����ֹͣ���� |
| B��������FeCl3��Һ������е�NaOH��Һ�У��Ӷ��Ƶ�Fe(OH)3���� |
| C�����˲��������������뽺�����Һ |
| D����ʼ����ʱ��Ӧ���ȼ���������ƿ���ٿ�����ˮ��������ϣ�Ӧ���ȹ�����ˮ�ٳ��ƾ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com