
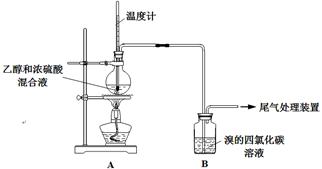
| �� �� | �� �� |
| ��ȼ�ƾ��ƣ� ������170�� | ��A����ƿ��Һ�彥����� ��B����������ð������Һ����ɫ |
| ���� | |
| ʵ����ϣ� ��ϴ��ƿ | ��A����ƿ�ڸ���������ɫ����״��д̼�����ζ�ݳ� |
| | �� �� | �� �� |
| �� | ��A��B������һ��װ��ij���Լ���ϴ��ƿ | Br2��CCl4��Һ��ɫ |
| �� | ��A���ӵ�װ�����£� | D����Һ�ɺ���ɫ��Ϊdz����ɫʱ��E����Һ��ɫ |
 ��2�֣�
��2�֣�

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д� ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ҵ�Ͻ���Mg��Al�����õ������Ӧ���Ȼ���ˮ��Һ�Ƶõ� |
| B����������Ʒ���渲��������Ĥ�����ڲ������𱣻����� |
| C��CO��NO��NO2���Ǵ�����Ⱦ���壬�ڿ����ж����ȶ����� |
| D��Ϊ�ⶨ�����������Ƶĵ����ԣ��ɽ��������ƹ������ʯӢ�����м����ۻ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����еı��ӣ�Br2ˮ�����ˣ� |
| B�����������е����ᣨ����̼������Һ����Һ�� |
| C���������е��Ҵ���ˮ����Һ�� |
| D���屽�е��壨NaOH��Һ����Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������NaHCO3��CO2 | B����Cu��ϡHNO3��Ӧ��NO |
| C����NH4Cl��ŨNaOH��Һ��Ӧ��NH3 | D����KMnO4��ŨHCl��Ӧ��Cl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
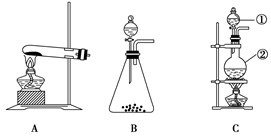
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
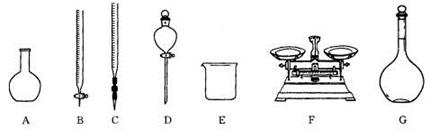
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com