
���Ṥ��β������ʱ��NaOH��Һ����SO2����NaHSO3������NaIO3�����з�Ӧ����ƽ������ȡ����I2��NaIO3��Դ����Ȼ��Ŀ��
�� NaIO3 + NaHSO3�� NaI + Na2SO4 + H2SO4 �� IO3����I����H����I2��H2O
��1����ƽ������Ӧ
��2���ں�5molNaHSO3����Һ����μ���NaIO3��Һ������㿪ʼ��������I2����������I2���ֵʱ�������NaIO3�����ʵ������Ƕ��٣�
��3��25��ʱ��H2SO3  HSO3-
+ H+�ĵ��볣��Ka= 1��10-2 mol/L������¶���NaHSO3��ˮ��ƽ�ⳣ��Kh=
mol/L������NaHSO3��Һ�м���������I2������Һ��
HSO3-
+ H+�ĵ��볣��Ka= 1��10-2 mol/L������¶���NaHSO3��ˮ��ƽ�ⳣ��Kh=
mol/L������NaHSO3��Һ�м���������I2������Һ�� �� ���������С�����䡱����ͬ���� ��������NaOH��Һ��
�� ���������С�����䡱����ͬ���� ��������NaOH��Һ�� ��ֵ
����������ˮ��ˮ�ĵ���̶Ƚ� ��
��ֵ
����������ˮ��ˮ�ĵ���̶Ƚ� ��
��1��2NaIO3 + 6NaHSO3 ��2NaI + 3Na2SO4 + 3H2SO4 IO3����5I����6H��= 3I2��3H2O
��2��5/3mol 2mol ��3��1.0��10-12��������������
��������
�����������1����������Ӧ��Ϊ������ԭ��Ӧ��Ҫ���ϵ����غ��ԭ���غ㡣�Եڶ�����Ӧ��˵�������ӷ�Ӧ����Ҫ���ϵ���غ㡣��ƽ��ķ���ʽΪ��2NaIO3 + 6NaHSO3 ��2NaI + 3Na2SO4 + 3H2SO4
IO3����5I����6H��= 3I2��3H2O ��2����ʼ����I2ʱ����һ����Ӧǡ����ɣ��ڶ�����Ӧ�ոտ�ʼ���з���ʽ2NaIO3 + 6NaHSO3 ��2NaI + 3Na2SO4 + 3H2SO4��֪��n(NaIO3):n(NaHSO3)=2:6=1:3,����n(NaIO3)=1/3 n(NaHSO3) =
1/3��5mol=5/3mol.��������I2���ֵʱ��һ����Ӧ������I-��ȫ�����ڶ�����Ӧ��n(I-)= 1/3n(NaHSO3)=
5/3 mol,�����ڶ�����Ӧ���ĵ�NaIO3�����ʵ���Ϊn(NaIO3)=1/5��5/3mol=1/3mol.����һ�����ĵĵ�NaIO3�����ʵ���Ϊn(NaIO3)=
5/3mol+1/3mol=2mol.��3�� H2SO3
 HSO3-
+ H+�ĵ��볣��Ka= {C(H+)��C(HSO3-)}
��C(H2SO3)= 1��10-2 mol/L; NaHSO3��ˮ��ƽ��ΪHSO3-+H2O
HSO3-
+ H+�ĵ��볣��Ka= {C(H+)��C(HSO3-)}
��C(H2SO3)= 1��10-2 mol/L; NaHSO3��ˮ��ƽ��ΪHSO3-+H2O H2SO3 + OH-��ˮ��ƽ�ⳣ��Kh=
{ C(OH-)��C(H2SO3)}��{ C(HSO3-)��C(H2O)}.
Ka��Kh= {C(H+)��C(OH-)}��C(H2O).
Kh= {C(H+)��C(OH-)} ��{C(H2O)��Ka
} =1.0��10-14��1.0��10-2=1.0��10-12. ����NaHSO3��Һ�м���������I2���ᷢ����Ӧ��H2SO3+ I2��H2O= H2SO4+ 2HI ��ʹƽ��H2SO3
H2SO3 + OH-��ˮ��ƽ�ⳣ��Kh=
{ C(OH-)��C(H2SO3)}��{ C(HSO3-)��C(H2O)}.
Ka��Kh= {C(H+)��C(OH-)}��C(H2O).
Kh= {C(H+)��C(OH-)} ��{C(H2O)��Ka
} =1.0��10-14��1.0��10-2=1.0��10-12. ����NaHSO3��Һ�м���������I2���ᷢ����Ӧ��H2SO3+ I2��H2O= H2SO4+ 2HI ��ʹƽ��H2SO3
 HSO3-
+ H+���淴Ӧ�����ƶ�������Һ��
HSO3-
+ H+���淴Ӧ�����ƶ�������Һ�� ����������������NaOH��Һ�����ڷ�����Ӧ��H++OH-=
H2O��ʹƽ��HSO3 -
����������������NaOH��Һ�����ڷ�����Ӧ��H++OH-=
H2O��ʹƽ��HSO3 - SO32- + H+������Ӧ�����ƶ���
SO32- + H+������Ӧ�����ƶ��� ��ֵ��������������ˮ���ε�ˮ��̶�����ʹˮ�ĵ���̶�Ҳ����
��ֵ��������������ˮ���ε�ˮ��̶�����ʹˮ�ĵ���̶�Ҳ����
���㣺�������Ṥ��β���������⼰����������ε�ˮ��ƽ�⡢����ƽ���Ӱ���֪ʶ��


 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪����HSO3-�Ļ�ԭ�Ա�I-ǿ����IO3-+5I-+6H+=3I2+3H2O����ҵ������NaIO3��NaHSO3��Ӧ����ȡ����I2��NaIO3��Դ����Ȼ��Ŀ��NaHSO3��Դ�����Ṥ��β�������IJ����NaOH��Һ����SO2����
��֪����HSO3-�Ļ�ԭ�Ա�I-ǿ����IO3-+5I-+6H+=3I2+3H2O����ҵ������NaIO3��NaHSO3��Ӧ����ȡ����I2��NaIO3��Դ����Ȼ��Ŀ��NaHSO3��Դ�����Ṥ��β�������IJ����NaOH��Һ����SO2�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�콭��������ѧ������ǰ����ѵ����ѧ���� ���ͣ�������
��������͵����������Ǵ�������Ҫ��Ⱦ���ֹ������������Ⱦ�ǵ�ǰ������������Ҫ�о�����֮һ��
��ij�¶��£�SO2��g����1/2O2��g��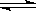 SO3��g������H����98 kJ��mol��1����ʼʱ��100 L���ܱ������м���4.0 mol SO2(g)��10.0 mol O2(g)������Ӧ�ﵽƽ��ʱ���ų�����196 kJ�����¶���ƽ�ⳣ��K�� �������£������������ټ���4mol SO2(g)�������´ﵽƽ��ʱSO2����ת���� ԭƽ��ʱSO2ת���ʣ�ѡ���������������������
SO3��g������H����98 kJ��mol��1����ʼʱ��100 L���ܱ������м���4.0 mol SO2(g)��10.0 mol O2(g)������Ӧ�ﵽƽ��ʱ���ų�����196 kJ�����¶���ƽ�ⳣ��K�� �������£������������ټ���4mol SO2(g)�������´ﵽƽ��ʱSO2����ת���� ԭƽ��ʱSO2ת���ʣ�ѡ���������������������
�� ��CH4����ԭNOxΪN2�������������������Ⱦ����д���ܷ�Ӧ����ʽ��
������1L NO2��NO�������NOx�����仹ԭ��N2����ͬ��ͬѹ��CH4�����0.4L������������NO2��NO�����ʵ���֮��Ϊ ��
�� ���Ṥ��β������ʱ��NaOH��Һ����SO2����NaHSO3������NaIO3�����з�Ӧ��δ��ƽ������ȡ����I2��NaIO3��Դ����Ȼ��Ŀ��
��NaIO3+NaHSO3��NaI+Na2SO4+H2SO4
��IO3����I����H����I2��H2O
�ں�5molNaHSO3����Һ����μ���NaIO3��Һ������㿪ʼ��������I2����������I2���ֵʱ�������NaIO3�����ʵ������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������͵����������Ǵ�������Ҫ��Ⱦ���ֹ������������Ⱦ�ǵ�ǰ������������Ҫ�о�����֮һ��
��ij�¶��£�SO2��g����1/2O2��g��![]() SO3��g������H����98 kJ��mol��1����ʼʱ��100 L���ܱ������м���4.0 mol SO2(g)��10.0 mol O2(g)������Ӧ�ﵽƽ��ʱ���ų�����196 kJ�����¶���ƽ�ⳣ��K�� �������£������������ټ���4mol SO2(g)�������´ﵽƽ��ʱSO2����ת���� ԭƽ��ʱSO2ת���ʣ�ѡ���������������������
SO3��g������H����98 kJ��mol��1����ʼʱ��100 L���ܱ������м���4.0 mol SO2(g)��10.0 mol O2(g)������Ӧ�ﵽƽ��ʱ���ų�����196 kJ�����¶���ƽ�ⳣ��K�� �������£������������ټ���4mol SO2(g)�������´ﵽƽ��ʱSO2����ת���� ԭƽ��ʱSO2ת���ʣ�ѡ���������������������
�� ��CH4����ԭNOxΪN2�������������������Ⱦ����д���ܷ�Ӧ����ʽ��
������1L NO2��NO�������NOx�����仹ԭ��N2����ͬ��ͬѹ��CH4�����0.4L������������NO2��NO�����ʵ���֮��Ϊ ��
�� ���Ṥ��β������ʱ��NaOH��Һ����SO2����NaHSO3������NaIO3�����з�Ӧ��δ��ƽ������ȡ����I2��NaIO3��Դ����Ȼ��Ŀ��
��NaIO3+NaHSO3��NaI+Na2SO4+H2SO4
��IO3����I����H����I2��H2O
�ں�5molNaHSO3����Һ����μ���NaIO3��Һ������㿪ʼ��������I2����������I2���ֵʱ�������NaIO3�����ʵ������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009�꽭��ʡ�����л�����ѧ�߿���ѧģ���Ծ��������������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com