
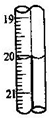
 ��������ƵĴ��ȿ��õζ������вⶨ��ԭ���ǣ�2S2O32-+I2��S4O62-+2I-
��������ƵĴ��ȿ��õζ������вⶨ��ԭ���ǣ�2S2O32-+I2��S4O62-+2I-| ��� | 1 | 2 | 3 |
| ����I2��Һ�����/mL | 19.98 | 20.02 |
| 1 |
| 600 |
| 1 |
| 600 |
| 50 |
| 10 |
| 1 |
| 120 |
| ||
| 2.500g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���۲���� | B������ |
| C�����ú�۲� | D�������ЧӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | �����Ϣ |
| X | X��̬ԭ���гɶԵ�����δ�ɶԵ�����֮��Ϊ4��3 |
| Y | Y��̬ԭ��L���������K���3�� |
| Z | ZԪ������Ȼ���д���������Ϊ35��37�����ֺ��� |
| W | WΪһ�ֳ������������γɶ��������������һ�־��д��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
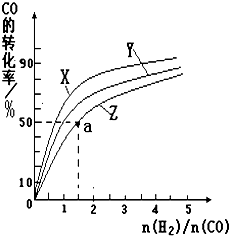 �״���һ������ȼ�ϣ��״�ȼ�ϵ�ؼ�����ʵ��������ҵ����������ҵ��һ����CO��H2Ϊԭ�Ϻϳɼ״����÷�Ӧ���Ȼ�ѧ����ʽΪ��
�״���һ������ȼ�ϣ��״�ȼ�ϵ�ؼ�����ʵ��������ҵ����������ҵ��һ����CO��H2Ϊԭ�Ϻϳɼ״����÷�Ӧ���Ȼ�ѧ����ʽΪ��| 1 |
| 2 |
| 1 |
| 2 |
| 0min | 5min | 10min | |
| CO | 0.1 | 0.05 | |
| H2 | 0.2 | 0.2 | |
| CH3OH | 0 | 0.04 | 0.05 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ij��Һ�м���Ba��NO3��2��Һ�������ɫ��������ԭ��Һ��һ������SO42- |
| B����ijϡ��Һ�м���Ba��NO3��2��Һ�������������ٵ��뼸�������ữ��AgNO3��Һ��������ɫ������˵��һ�����Ȼ������Һ |
| C����ʢ��H2��С�Թܹܿ����Ͽ����ƾ��ƻ������H2�Ĵ��� |
| D����ȼ�ŵ�ľ���������ܿڣ�ľ��Ϩ��˵����CO2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com