
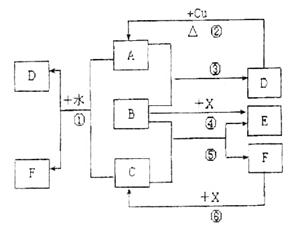
��10�֣���֪A~F����ѧ��ѧ���������ʣ�����A��C��EΪ���壬B��DΪҺ�壬D��һ�ֲ��ӷ����ᣬ��Ũ��Һ��ǿ�����ԣ�F����Һ��X����ͨ������ʵ�����Ʊ�����C��X��һ�ֺ�ɫ��ĩ��B��������18�����ӣ�ʵ���ҳ���B��X�Ʊ�����E����Ӧ�в�������������ȥ���Իش��������⣺
��1��д��B�ĵ���ʽ ����ѧ������ ��
��2������ͼ����Ϣ��B��C��X�����Դ�ǿ������˳����________________________��
��3��X���������·�Ӧ�ķ���ʽΪ______________��
��4��д����Ӧ�ڵĻ�ѧ����ʽ_____________��
��5����Ӧ�ٵ����ӷ���ʽ ��
��1�� �����Լ��ͷǼ��Լ���
�����Լ��ͷǼ��Լ���
��2��X��C��B�����MnO2��Cl2��H2O2��
��3��3MnO2+4Al 3Mn+2Al2O3
3Mn+2Al2O3
��4��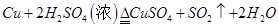
��5��Cl2+SO2+2H2O=4H++SO42-+2Cl-
��������
���������F�����壬�����γ�Ũ��Һ��˵��F��������ˮ����ѧ��ֻ�����ǰ������Ȼ��⣬����ΪF��Ũ��Һ�����ɫ��ĩX��Ӧ����ʵ�����Ʊ���������C��ʵ�������ú�ɫ��ĩ����Һ��Ӧ�Ʊ�������ֻ����������ϰ�ˮ����������ʣ���֪��C��Cl2��F��HCl��X��MnO2��������֪A��SO2��B��H2O2��E��O2��DΪ���ᡣȻ�����������ԭ��Ӧ�������������������������ǿ����֪�𰸣�
���㣺������ƶ�
����������Ҫ�����������ʵ���������Ѱ��ͻ�ƿڣ���D��һ�ֲ��ӷ����ᣬ��Ũ��Һ��ǿ�����ԣ���DΪ���ᡣ�����Ҫ���ڶ�����ʺͻ�ѧ��Ӧ��ɸѡ�������������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
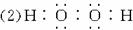
(1)������ͼ�е���Ϣ��B��C��D��X�����Դ�ǿ������˳����________________________��
(�ö�Ӧ���ʵĻ�ѧʽ��ʾ)
(2)B�ĵ���ʽΪ________________________ ��
(3)д����Ӧ�ڵĻ�ѧ����ʽ______________________________________________��
(4)д����Ӧ�٢����ӷ���ʽ��
��______________________________________________________________��
��______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ��������УЭ���������������Ͽ��Ի�ѧ�Ծ����������� ���ͣ������
��10�֣���֪A~F����ѧ��ѧ���������ʣ�����A��C��EΪ���壬B��DΪҺ�壬D��һ�ֲ��ӷ����ᣬ��Ũ��Һ��ǿ�����ԣ�F����Һ��X����ͨ������ʵ�����Ʊ�����C��X��һ�ֺ�ɫ��ĩ��B��������18�����ӣ�ʵ���ҳ���B��X�Ʊ�����E����Ӧ�в�������������ȥ���Իش��������⣺
��1��д��B�ĵ���ʽ ����ѧ������ ��
��2������ͼ����Ϣ��B��C��X�����Դ�ǿ������˳����________________________��
��3��X���������·�Ӧ�ķ���ʽΪ______________��
��4��д����Ӧ�ڵĻ�ѧ����ʽ_____________��
��5����Ӧ�ٵ����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com