
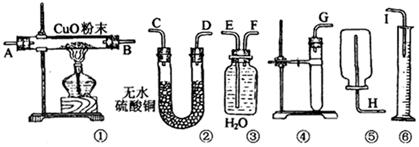
 ЕФЗНЗЈЪЧ ЁЃ
ЕФЗНЗЈЪЧ ЁЃ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЪЕбщЬт
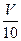 mLЃЌвдЗгЬЊзїжИЪОМСЃЌгУcЁф mol/L NaOHШмвКЕЮЖЈЃЌЧЁКУгУШЅVЁфmLЁЃ
mLЃЌвдЗгЬЊзїжИЪОМСЃЌгУcЁф mol/L NaOHШмвКЕЮЖЈЃЌЧЁКУгУШЅVЁфmLЁЃ| AЃЎcЁЂV | BЃЎcЁфЁЂ VЁф | CЃЎ MЁф | DЃЎ M |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЪЕбщЬт

| AЃЎЯЁЯѕЫс | BЃЎЫЋбѕЫЎ | CЃЎАБЫЎ | DЃЎИпУЬЫсМиШмвК |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЬюПеЬт
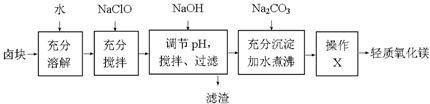
| Юя жЪ | ПЊЪМГСЕэЪБЕФpH | ГСЕэЭъШЋЪБЕФpH |
| Fe(OH)3 | 2.7 | 3.7 |
| Fe(OH)2 | 7.6 | 9.6 |
| Mn(OH)2 | 8.3 | 9.8 |
| Mg(OH)2 | 9.6 | 11.1 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЬюПеЬт
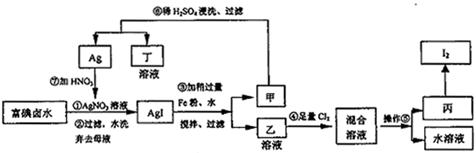
 ПЩвдбЛЗРћгУЕФИБВњЮяЪЧ ЁЃ
ПЩвдбЛЗРћгУЕФИБВњЮяЪЧ ЁЃВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЪЕбщЬт
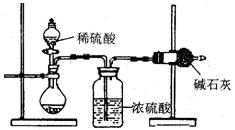
| AЃЎзАжУФкдгаПеЦјжаЕФЖўбѕЛЏЬМЦјЬхвВБЛМюЪЏЛвЮќЪе |
| BЃЎзАжУЭтПеЦјжаЕФЫЎеєЦјКЭЖўбѕЛЏЬМБЛМюЪЏЛвЮќЪе |
| CЃЎЗДгІЭъГЩКѓЃЌзАжУжаЕФЖўбѕЛЏЬМУЛгаШЋВПБЛМюЪЏЛвЮќЪе |
| DЃЎМгШыЯЁСђЫсЕФСПВЛзуЃЌдђПЩФмдьГЩЮѓВю |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
| AЃЎЙ§ТЫЪБВЃСЇАєФЉЖЫЧсЧсЕиППдкШ§ВуТЫжНЩЯ |
| BЃЎеєСѓЪБРфФ§ЫЎДгРфФ§ЙмЯТПкНјЩЯПкГі |
| CЃЎЗжвКВйзїЪБЗжвКТЉЖЗжаЯТВувКЬхДгЯТПкЗХГіЃЌЩЯВувКЬхДгЩЯПкЕЙГі |
| DЃЎнЭШЁВйзїЪБЃЌгІбЁдёгаЛњнЭШЁМСЃЌЧвнЭШЁМСЕФУмЖШБиаыБШЫЎДѓ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
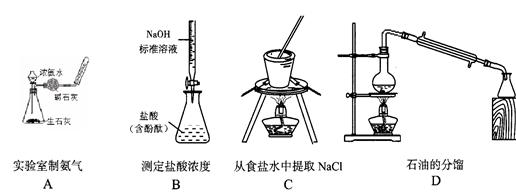
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКВЛЯъ ЬтаЭЃКЕЅбЁЬт
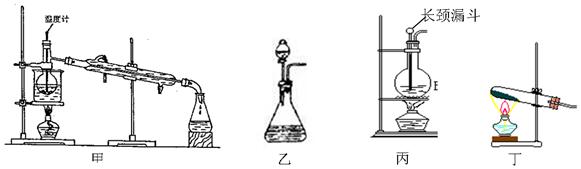
| AЃЎзАжУМзЃКЪЏгЭЗжСѓ | BЃЎзАжУввЃКфхБНЕФжЦШЁ |
| CЃЎзАжУБћЃКввЯЉЕФжЦШЁ | DЃЎзАжУЖЁЃКввЫсввѕЅЕФжЦШЁ |
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com