

���� ú����Ҫ����Al2O3��SiO2����ú���м���ϡ���ᣬ������ӦAl2O3+6H+=2Al3++3H2O��SiO2����Ӧ��Ȼ����ù��˷����õ�����1ΪSiO2����Һ������Ϊϡ�����Al2��SO4��3��������ҺpHΪ3.6��Ȼ�����CaCO3��ĩ��������ӦCaCO3+2H+�TCa2++CO2��+H2O��CaSO4Ϊ�����������2�ijɷ���ҪΪCaSO4�����˵���Һ��
��1�����������Թ������Һ���ù��˷�����
��2����������������������ܺ�ϡ���ᷴӦ�����κ�ˮ��Ϊ��������ʱ��Ԫ�صĽ����ʣ����Բ���������ҺŨ�ȡ������¶Ȼ�����Ӧ��Ӵ�����ȷ�����
��3������2�Ļ�ѧʽ��CaSO4��������Ũ��������c��Ca2+��•c��SO42-����Ksp��CaSO4����
��4��Al2��SO4��3•2Al��OH��3��SO2��Ӧ����Al2��SO4��3•Al2��SO3��3��Al2��SO4��3•Al2��SO3��3��O2��������Al2��SO4��3��
��� �⣺ú����Ҫ����Al2O3��SiO2����ú���м���ϡ���ᣬ������ӦAl2O3+6H+=2Al3++3H2O��SiO2����Ӧ��Ȼ����ù��˷����õ�����1ΪSiO2����Һ������Ϊϡ�����Al2��SO4��3��������ҺpHΪ3.6��Ȼ�����CaCO3��ĩ��������ӦCaCO3+2H+�TCa2++CO2��+H2O��CaSO4Ϊ�����������2�ijɷ���ҪΪCaSO4�����˵���Һ��
��1�����������Թ������Һ���ù��˷�����������������ϡ���ᡢ��������������ϡ���ᣬ���Բ��ù��˷������룬�ʴ�Ϊ�����ˣ�
��2����������������������ܺ�ϡ���ᷴӦ�����κ�ˮ�����ӷ�Ӧ����ʽΪAl2O3+6H+=2Al3++3H2O��Ϊ��������ʱ��Ԫ�صĽ����ʣ����Բ���������ҺŨ�ȡ������¶Ȼ�����Ӧ��Ӵ�����ȷ�����
�ʴ�Ϊ��Al2O3+6H+=2Al3++3H2O����ú�����飬���裨ʹ��ú����������Һ��ֽӴ������ʵ��ӳ������ʱ�䣬��������Ũ�ȣ������¶ȣ�
��3������2�Ļ�ѧʽ��CaSO4��CaCO3+2H+=Ca2++H2O+CO2����������Ca2+Ũ�ȣ�ʹc��Ca2+��•c��SO42-����Ksp��CaSO4��������Ca2++SO42-=CaSO4�� ��CaCO3+2H+=Ca2++H2O+CO2�����ٽ�CaCO3��s�������ܽ�ƽ�����ܽⷽ���ƶ���������Ca2+Ũ�ȣ�ʹc��Ca2+��•c��SO42-����Ksp��CaSO4�����ٽ�CaSO4��s�������ܽ�ƽ������������ƶ���
�ʴ�Ϊ��CaSO4��CaCO3+2H+=Ca2++H2O+CO2����������Ca2+Ũ�ȣ�ʹc��Ca2+��•c��SO42-����Ksp��CaSO4��������Ca2++SO42-=CaSO4�� ��CaCO3+2H+=Ca2++H2O+CO2�����ٽ�CaCO3��s�������ܽ�ƽ�����ܽⷽ���ƶ���������Ca2+Ũ�ȣ�ʹc��Ca2+��•c��SO42-����Ksp��CaSO4�����ٽ�CaSO4��s�������ܽ�ƽ������������ƶ���
��4��Al2��SO4��3•2Al��OH��3��SO2��Ӧ����Al2��SO4��3•Al2��SO3��3��Al2��SO4��3•Al2��SO3��3��O2��������Al2��SO4��3����Ӧ����ʽΪAl2��SO4��3•2Al��OH��3+3SO2=Al2��SO4��3•Al2��SO3��3+3H2O��2Al2��SO4��3•Al2��SO3��3+3O2=4Al2��SO4��3��
�ʴ�Ϊ��Al2��SO4��3•2Al��OH��3+3SO2=Al2��SO4��3•Al2��SO3��3+3H2O��2Al2��SO4��3•Al2��SO3��3+3O2=4Al2��SO4��3��
���� ���⿼�����ʷ�����ᴿ��Ϊ��Ƶ���㣬���ؿ������ӷ�Ӧ���������ܽ�ƽ�⼰������������ȷ���ʵ����ʼ����������������ɽ���ѵ��ǣ�3���⣬֪��Ũ�Ȼ����ܶȻ��Ĺ�ϵ����Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c��NH4+��=2c��SO42-����c��H+��=c��OH-�� | B�� | c��NH4+����c��SO42-����c��H+����c��OH-�� | ||
| C�� | c��SO42-����c��NH4+����c��H+����c��OH-�� | D�� | c��SO42-����c��NH4+����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
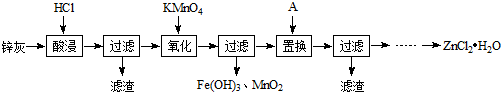
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
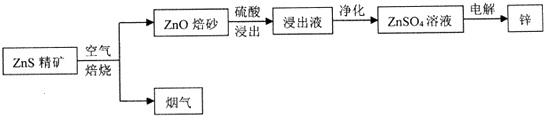
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Fe��OH��3 | Fe��OH��2 | Cu��OH��2 | |
| ��ʼ����ʱ��pH | 2.7 | 7.6 | 5.2 |
| ��ȫ����ʱ��pH | 3.7 | 9.6 | 6.4 |
| ��1 | |||
| ��� | 1 | 2 | 3 | 4 | |
| �������/mL | 25.05 | 25.00 | 26.80 | 24.95 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
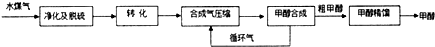
| ���� | H2 | CO | CH3OH |
| Ũ��/��mol•L-1�� | 0.2 | 0.1 | 0.4 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ����NaOH��AlCl3��MgCl2���ֹ�����ɵĻ������������ˮ�У���0.58g��ɫ���������������õĻ���Һ�У�����1mol/L���ᣬ�����������������ɳ�����������ϵ��ͼ��ʾ����������NaOH����Ϊ��������
����NaOH��AlCl3��MgCl2���ֹ�����ɵĻ������������ˮ�У���0.58g��ɫ���������������õĻ���Һ�У�����1mol/L���ᣬ�����������������ɳ�����������ϵ��ͼ��ʾ����������NaOH����Ϊ��������| A�� | 3.6g | B�� | 4g | C�� | 4.4g | D�� | 4.8g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
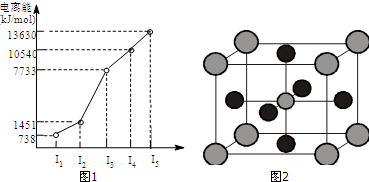
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ��������a��b��c��d | |
| B�� | ���Ӱ뾶��A2+��B+��C2-��D- | |
| C�� | ���ʻ�ԭ�ԣ�B��A�����������ԣ�D��C | |
| D�� | ���ӻ�ԭ�ԣ�C2-��D-�����������ԣ�B+��A2+ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com