
��������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����á���ҵ������ú���������ˮú�����ϳɶ����ѡ�
��ش��������⣺
��1��ú����������Ҫ��ѧ����ʽΪ ��
��2������ˮú���ϳɶ����ѵ��ܷ�Ӧʽ�ɱ�ʾΪ3CO(g)��3H2(g) CH3OCH3(g)��CO2(g) ��H�� 0��һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ�� ������ĸ���ţ���
CH3OCH3(g)��CO2(g) ��H�� 0��һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ�� ������ĸ���ţ���
| A�����¸�ѹ | B��������� |
| C������CO2��Ũ�� | D������CO��Ũ�� E������������� |
 CH3OCH3(g)��H2O(g),ij�¶��µ�ƽ�ⳣ��Ϊ400.���¶��£����ܱ������м���CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
CH3OCH3(g)��H2O(g),ij�¶��µ�ƽ�ⳣ��Ϊ400.���¶��£����ܱ������м���CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol/L�� | 0.44 | 0.6 | 0.6 |
��10�֣�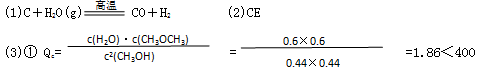
����������У� v(��) �� v���棩
�� 2CH3OH(g)  CH3OCH3(g)��H2O(g)
CH3OCH3(g)��H2O(g)
ʼ̬��mol/L�� c0 0 0
ijʱ�� 0.44 0.6 0.6
z��ߵ� 0.44+1.2 0 0 c��CH3OH��=0.44+1.2=1.64mol/L
��ƽ�ⷴӦŨ�� ��c ��c/2 ��c/2
ƽ��Ũ�� 0.44-��c ��c/2 ��c/2
��c ="1.6mol/L " ƽ�⣬ʱCH3OH�����ʵ���Ũ�� 0.04mol/L
��ʱ����CH3OH�Ļ�ѧ��Ӧ����Ϊ0.16mol/��L��min��
����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol?L-1�� | 0.44 | 0.6 | 0.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?�������ģ����������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ������H2��CO2�ϳɶ����ѵķ�Ӧ���£�6H2��g��+2CO2��g��?CH3OCH3��g��+3H2O��g��
��2012?�������ģ����������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ������H2��CO2�ϳɶ����ѵķ�Ӧ���£�6H2��g��+2CO2��g��?CH3OCH3��g��+3H2O��g��| 0.03 |
| t |
| 0.03 |
| t |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol?L-1�� | 0.44 | 0.6 | 0.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
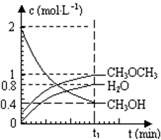 ��֪��������һ����Ҫ�����ȼ�ϣ�����ͨ��CH3OH���Ӽ���ˮ�Ƶã�2CH3OH��g��?CH3OCH3��g��+H2O��g����H=23.5kJ?mol-1����T1�棬�����ܱ������н�������ƽ�⣬��ϵ�и����Ũ����ʱ��仯��ͼ��ʾ����ش��������⣺
��֪��������һ����Ҫ�����ȼ�ϣ�����ͨ��CH3OH���Ӽ���ˮ�Ƶã�2CH3OH��g��?CH3OCH3��g��+H2O��g����H=23.5kJ?mol-1����T1�棬�����ܱ������н�������ƽ�⣬��ϵ�и����Ũ����ʱ��仯��ͼ��ʾ����ش��������⣺| c(CH3OCH3)?c(H2O) |
| c2(CH3OH) |
| c(CH3OCH3)?c(H2O) |
| c2(CH3OH) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��ѧ�� | C-H | O-H | H-H | C-O | C��O |
| ����/kJ��mol-1 | 413 | 463 | 436 | 358 | 1072 |
| ���� | CH3OH ��g�� | CH3OCH3 ��g�� | H2O ��g�� |
| ��ʼŨ��/mol��l-1 | 2 | 0.2 | 0 |
| ƽ��Ũ��/mol��l-1 | 0.4 | 1 | 0.8 |
| ���� | CH3OH ��g�� | CH3OCH3 ��g�� | H2O ��g�� |
| ��ʼŨ��/mol��l-1 | 0.4 | 1.2 | 0.6 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com