
��֪2SO2(g)+O2(g) 2SO3(g)����H= -197kJ/mol��ͬ���£�����ͬ������ܱ������ס����зֱ����2molSO2��1molO2��1molSO3��1molSO2��0.5molO2�������Ƿֱ�ﵽƽ��ʱ�ų�������ΪQ1KJ��Q2KJ�������бȽ���ȷ����
2SO3(g)����H= -197kJ/mol��ͬ���£�����ͬ������ܱ������ס����зֱ����2molSO2��1molO2��1molSO3��1molSO2��0.5molO2�������Ƿֱ�ﵽƽ��ʱ�ų�������ΪQ1KJ��Q2KJ�������бȽ���ȷ����
| A��Q2=1/2Q1=98.5 | B��Q2=1/2Q1<98.5 | C��Q2<1/2Q1<98.5 | D����ȷ�� |
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��25�桢101kPa�£� 1g������ȫȼ������CO2��Һ̬H2O���ų�55 kJ��������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ�� ��
(2)2Zn��s��+O2��g��=2ZnO��s�� ��H1 = ��702 kJ/mol
2Hg��l��+O2��g��=2HgO��s�� ��H2 = ��182 kJ/mol
�ɴ˿�֪ZnO��s��+Hg��l��= Zn��s��+HgO��s����H3= ��
��3����֪ 2SO2(g)+O2(g)=2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2(g)����Ϊ1mol SO3�Ħ�H=��99kJ��mol-1����ش��������⣺
��ͼ��A��C�ֱ��ʾ �� ��
��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿 ���÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B�������ǽ��ͣ� �������� ����������������
��ͼ�С�H= kJ��mol-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣���֪2SO2 (g)+ O2(g)2SO3(g) ��H =��196kJ��mol-1��5000Cʱ��2mol SO2��1molO2װ��һ����㶨��10L�ܱ������У�2���ӣ�t1��ʱ�ﵽƽ�⡣����ͼ��ʾ���߿�
��ش��������⣺
��1����O2��ʾ2�����ڸ÷�Ӧ��ƽ����Ӧ����Ϊ mol��L-1��min-1������500��ʱ�÷�Ӧ��ƽ�ⳣ��K= ��
��2����ͼ��ʾ�÷�Ӧ��ʱ��t1�ﵽƽ���ʱ��t2��ı�ij�������������仯�������
ͼ��ʱ��t2�����ı������������ ��дһ�����ɣ���
��3����������������ʼװ���SO2��O2��2mol����ƽ�����SO2��ת����Ϊx���г���x�ķ��� �����������x��
��4��ij�¶�ʱ���÷�Ӧ��ƽ�ⳣ��K=5000������¶� 500�棨�������������������������
��5��500��ʱ������ʼװ���SO2��O2��SO3�ֱ�Ϊ0.2mol��ymol��wmol���ﵽƽ��ʱ����ֵĺ�����ڣ�3����ȫ��ͬ����y= mol���տ�ʼʱ��Ӧ�� ����������桱����Ӧ������С�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ�����IJ���ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ���ѡ��
���������Ȼ�ѧ����ʽ�ó��Ľ�����ȷ���ǣ� ��
A����֪2SO2(g)+O2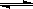 2SO3(g) Ϊ���ȷ�Ӧ����SO2������һ������SO3������ 2SO3(g) Ϊ���ȷ�Ӧ����SO2������һ������SO3������ |
| B����֪C(ʯī,s) ===C�����ʯ,s�� ��H��0������ʯ��ʯī�ȶ� |
| C��NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)����H����57.4 kJ/mol����20 g NaOH����Һ������ϡ������ȫ��Ӧ���ų�������Ϊ28.7 kJ |
| D����֪2C(s) +2O2 (g) ===2CO2(g) ��H1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ����������������һ�и߶���һ���¿���ѧ�Ծ����������� ���ͣ������
��10�֣���֪��a��H+(aq) + OH-(aq) = H2O(l) ��H=-57.3 kJ?mol-1��
b��1.6gCH4��ȫȼ������ˮ����ʱ����80.2kJ��1gˮ����ת����Һ̬ˮ����2.444kJ��
��1������������������ϡ��Һ������Ӧ��д���������к��ȵ��Ȼ�ѧ����ʽ��
��2��д����������ȼ���ȵ��Ȼ�ѧ����ʽ��
��3����֪2SO2(g)+O2(g)  2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺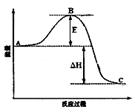
��ͼ��C��ʾ E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿 ��
�ڸ÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B�������ǽ��ͣ� �������� ��
��4����˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ�������3���Ȼ�ѧ��Ӧ����ʽ��
��Fe2O3(s)+3CO(g)=2Fe(s)+3CO2(g) ��H���D24.8 kJ?mol-1
�� 3Fe2O3(s)+ CO(g)=2Fe3O4(s)+ CO2(g) ��H���D47.2 kJ?mol-1
��Fe3O4(s)+CO(g)=3FeO(s)+CO2(g) ��H�� +640.5 kJ?mol-1
д��CO���廹ԭFeO����õ�Fe�����CO2������Ȼ�ѧ��Ӧ����ʽ��
_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪2SO2(g)+O2(g)  2SO3(g)(����Ӧ����)������500��ʹ����������£��÷�Ӧ���ݻ��̶����ܱ������н��У������й�˵����ȷ����
2SO3(g)(����Ӧ����)������500��ʹ����������£��÷�Ӧ���ݻ��̶����ܱ������н��У������й�˵����ȷ����
A���������¶ȣ����Լӿ췴Ӧ����
B��ʹ�ô�����Ϊ�˼ӿ췴Ӧ����
C�������������£�SO2����ȫת��ΪSO3
D���ﵽƽ��ʱ��SO2��SO3��Ũ��һ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com