
NH3��һϵ�з�Ӧ���Եõ�HNO3��NH4NO3������ͼ��ʾ��
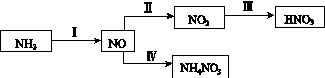
(1)���У�NH3��O2�ڴ��������·�Ӧ���仯ѧ����ʽ��_________________________��
(2)����2NO(g)��O2(g)  2NO2(g)��������������
2NO2(g)��������������
ͬʱ���ֱ���NO��ƽ��ת�����ڲ�ͬѹǿ(p1��p2)�����¶ȱ仯������(��ͼ)��

�ٱȽ�p1��p2�Ĵ�С��ϵ��________��
�����¶����ߣ��÷�Ӧƽ�ⳣ���仯��������________��
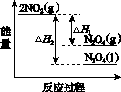
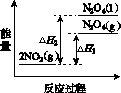
(3)���У������¶ȣ���NO2(g)ת��ΪN2O4(l)�����Ʊ�Ũ���ᡣ
����֪��2NO2(g)  N2O4(g)����H1 2NO2(g)
N2O4(g)����H1 2NO2(g) N2O4(l)����H2
N2O4(l)����H2
���������仯ʾ��ͼ�У���ȷ����(ѡ����ĸ)________��
 ��
��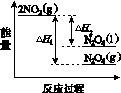
������A������������������B C
��N2O4��O2��H2O���ϵĻ�ѧ����ʽ��________________________________________��
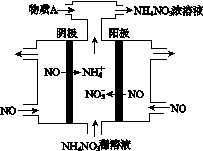
(4)���У����NO�Ʊ�NH4NO3���乤��ԭ����ͼ��ʾ��Ϊʹ������ȫ��ת��ΪNH4NO3���貹��A��A��________��˵�����ɣ�________________________________________��
(1)4NH3��5O2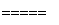 4NO��6H2O
4NO��6H2O
(2)��p1��p2���ڼ�С
(3)��A����2N2O4��O2��2H2O===4HNO3
(4)NH3�����ݷ�Ӧ��8NO��7H2O 3NH4NO3��2HNO3����������HNO3��
3NH4NO3��2HNO3����������HNO3��
[����] (1)���Ĵ������ķ�Ӧ����ʽΪ
4NH3��5O2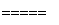 4NO��6H2O��
4NO��6H2O��
(2)����2NO(g)��O2(g)  2NO2(g)��֪�÷�ӦΪ���������С�ķ�Ӧ���¶���ͬ������ѹǿ��ƽ�������ƶ���NO��ƽ��ת����������ͼʾ�����꺬�壬�ж�p1<p2�����ٿ�ͬһѹǿ�ߣ��¶����ߣ�NO��ƽ��ת���ʽ��ͣ�ƽ�����淴Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ���¶����ߣ�ƽ�ⳣ����С��
2NO2(g)��֪�÷�ӦΪ���������С�ķ�Ӧ���¶���ͬ������ѹǿ��ƽ�������ƶ���NO��ƽ��ת����������ͼʾ�����꺬�壬�ж�p1<p2�����ٿ�ͬһѹǿ�ߣ��¶����ߣ�NO��ƽ��ת���ʽ��ͣ�ƽ�����淴Ӧ�����ƶ���������ӦΪ���ȷ�Ӧ���¶����ߣ�ƽ�ⳣ����С��
(3)�ٽ����¶ȣ�NO2(g)ת��ΪN2O4(l)����H2<0����Ӧ����������������������C���� N2O4(g)ת��ΪN2O4(l)��Ҫ�ų�����������NO2(g)ת��ΪN2O4(g)��NO2(g)ת��ΪN2O4(l)�ų��������٣�B����������ȷ��ͼʾΪA����N2O4��O2��H2O��Ӧ��������ķ�Ӧ����ʽΪ2N2O4�� O2��2H2O===4HNO3��
(4)���ݹ���ԭ��װ��ͼ������ȷ������ΪNOʧȥ����ת��ΪNO ������NOת��ΪNH
������NOת��ΪNH �����ݵ缫��Ӧ��д�缫��ӦʽΪ��
�����ݵ缫��Ӧ��д�缫��ӦʽΪ��
������NO��3e����2H2O===NO ��4H��
��4H��
������NO��5e���� 6H��===NH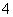 �� H2O
�� H2O
Ȼ����ݵ�ʧ�����غ㣬������������ʵ�����笠��������ʵ����࣬������Ҫ����Һ�м��������ΪNH3(��8NO��7H2O 3NH4NO3��2HNO3)��
3NH4NO3��2HNO3)��


 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������AX3�͵���X2��һ�������·�Ӧ�����ɻ�����AX5���ش��������⣺
(1)��֪AX3���۵�ͷе�ֱ�Ϊ��93.6 ���76 �棬AX5���۵�Ϊ167 �档����ʱAX3������X2��Ӧ����1 mol AX5���ų�����123.8 kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ____________________________________________��
(2)��ӦAX3(g)��X2(g)  AX5(g)���ݻ�Ϊ10 L���ܱ������н��С���ʼʱAX3��X2��Ϊ0.2 mol����Ӧ�ڲ�ͬ�����½��У���Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ��
AX5(g)���ݻ�Ϊ10 L���ܱ������н��С���ʼʱAX3��X2��Ϊ0.2 mol����Ӧ�ڲ�ͬ�����½��У���Ӧ��ϵ��ѹǿ��ʱ��ı仯��ͼ��ʾ��
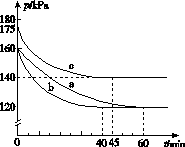
����ʽ����ʵ��a�ӷ�Ӧ��ʼ���ﵽƽ��ʱ�ķ�Ӧ����v(AX5)��______________________��
��ͼ��3��ʵ��ӷ�Ӧ��ʼ���ﵽƽ��ʱ�ķ�Ӧ����v(AX5)�ɴ�С�Ĵ���Ϊ____________(��ʵ�����)����ʵ��a��ȣ���������ı��ʵ���������ж������ǣ�b________________________________________________��c____________________________________________��
����p0��ʾ��ʼʱ��ѹǿ��p��ʾƽ��ʱ��ѹǿ������ʾAX3��ƽ��ת���ʣ������ı���ʽΪ______________��ʵ��a��c��ƽ��ת���ʣ���aΪ________����cΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�˵����ȷ����(����)
A�����ں�������ϸ���һЩͭ�飬����Լ���������ǵĸ�ʴ
B��2NO(g)��2CO(g)===N2(g)��2CO2(g)�ڳ��������Է����У���÷�Ӧ�Ħ�H>0
C������0.1 mol��L��1Na2CO3��Һ��CO ��ˮ��̶Ⱥ���Һ��pH������
��ˮ��̶Ⱥ���Һ��pH������
D�������������Ҵ���������Ӧ(��H<0)����������Ũ���Ტ���ȣ��÷�Ӧ�ķ�Ӧ���ʺ�ƽ�ⳣ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ת������Դ���úͻ�����������Ҫ�о����⡣�������������ж��ַ�����
(1)���ռ�����H2S�����Һ���뵽��ͼ��ʾ�ĵ��ص����������е�⡣���������������������·�Ӧ��
S2����2e��===S��(n��1)S��S2��===S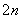
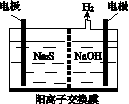
��д�����ʱ�����ĵ缫��Ӧʽ��________________��
�ڵ�������������Һ��ϡ�����ữ�õ����ʣ������ӷ���ʽ��д��__________________________��
(2)��H2S�Ϳ����Ļ������ͨ��FeCl3��FeCl2��CuCl2�Ļ����Һ�з�Ӧ����S��������ת����ͼ��ʾ��
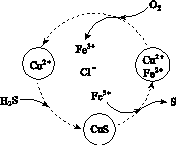
����ͼʾ��ת���У����ϼ۲����Ԫ����________��
�ڷ�Ӧ�е���1 mol H2Sת��Ϊ����ʱ��������Һ��Fe3�������ʵ������䣬������O2�����ʵ���Ϊ________��
�����¶�һ���Ͳ�������Һ�������£�����ͨ�������壬����ֽ��衣��ʹ���ɵ������в���CuS���ɲ�ȡ�Ĵ�ʩ��________________��
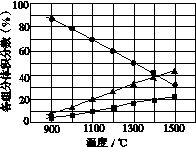
(3)H2S�ڸ����·ֽ�������������H2������Ӧ�ڲ�ͬ�¶��´ﵽƽ��ʱ����������и���ֵ����������ͼ��ʾ��H2S�ڸ����·ֽⷴӦ�Ļ�ѧ����ʽΪ__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
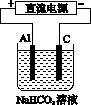
ijͬѧ��װ����ͼ��ʾ�ĵ绯ѧװ�ã��缫��ΪAl�������缫��ΪCu����(����)
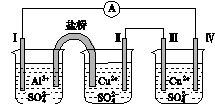
ͼ4
A���������缫���D�� �D���缫��
�D���缫��
B���缫������ԭ��Ӧ
C���缫�����ܽ�
D���缫��ĵ缫��Ӧ��Cu2����2e��===Cu
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij���صķ�ӦΪNiO2��Fe��2H2O Fe(OH)2��Ni(OH)2��
Fe(OH)2��Ni(OH)2��
(1)�����س��ʱ��������ԭ��Ӧ��������________(��������ĸ)���ŵ�ʱ����Fe(OH)2������Ϊ18 g�������·��ת�Ƶĵ�������____________________��
A��NiO2 B��Fe
C��Fe(OH)2 D��Ni(OH)2
(2)Ϊ��ֹԶ���ִ��ĸ��������ں�ˮ�з����绯ѧ��ʴ��ͨ���ڴ�����ǶZn�飬��������ص�______��(���������)������
ͼK197
(3)�Ը���������Դ������ͼK197��ʾ��װ����ʵ����ģ������Ʒ���桰�ۻ��������Ĺ����У�������Һ����ǣ�ԭ����(����صĵ缫��Ӧʽ�����ӷ���ʽ��ʾ)________________________________________________________________________
________________________________________________________________________��
(4)����ͭʱ����ͭӦ��ֱ����Դ��______��(���������)���������������У��������Һ�е�c(Fe2��)��c(Zn2��)���������Ӱ���һ����⡣��ͬѧ������³��ӷ�����



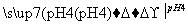

��֪��
| ������ | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 | Zn(OH)2 |
| ��ʼ����ʱ��pH | 2.3 | 7.5 | 5.6 | 6.2 |
| ��ȫ����ʱ��pH | 3.9 | 9.7 | 6.4 | 8.0 |
�����H2O2��Ŀ����________________________________________________________________________��
��ͬѧ��ΪӦ�������е�pH���ڵ�8������Ϊ�˹۵�____________(���ȷ������ȷ��)��������______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ǵ��ճ������г����ˡ��ӵ�ʳ�Ρ������������͡������߸�ţ�̡�����������Ҷ�������� ��
���ࡱ����Ʒ������ĵ⡢�����ơ�������Ӧ����Ϊ
A��Ԫ�� B������ C������ D��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ں����þ��Ϻ�DZˮͧ����Ϊ��������Դ�������� ��
A��Na2O2 B��NaHCO3 C��SO3(��) D��KMnO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Y��Z��W��Ϊ������Ԫ�أ����������ڱ��е�λ����ͼ��ʾ����֪Yԭ�ӵ������������Ǵ�����������3��������˵������ȷ����(����)
A��ԭ�Ӱ뾶��W��Z��Y��X
B������������Ӧˮ��������ԣ�Z��W��X
C���⻯����ȶ��ԣ�X��Y��Z
D������Ԫ�صĵ����У�Z���ʵ��ۡ��е����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com