
����Ŀ���㽭ʦ����ѧ���о��Ŷ��о������һ����и��Ժ�ѡ���Եĸ��ѿ�������![]() ������һ�ɹ�������2020��1��Chem. Mater.�ϡ����и���ɵ�CsPbBr3���������
������һ�ɹ�������2020��1��Chem. Mater.�ϡ����и���ɵ�CsPbBr3���������![]() �̶�������棬���ڿɼ�������CO2��ԭ��
�̶�������棬���ڿɼ�������CO2��ԭ��

(1)д��Ni��̬ԭ�ӵļ۵����Ų�ͼ���������ʽ��___________________��
(2)C��N��O��Cs��PbԪ�ص�һ�������ɴ�С��˳����____________________��
(3)![]() �Ƕ��������ӣ��������е�ԭ�ӵ��ӻ�����Ϊ_________�������ӽṹ�к���_________������ĸ����
�Ƕ��������ӣ��������е�ԭ�ӵ��ӻ�����Ϊ_________�������ӽṹ�к���_________������ĸ����
a�����Ӽ� b����λ�� C������ d.���
(4)ijЩ��������۵��������±���ʾ��
������ | CO2 | Cs2O | PbO |
�۵�/�� | -56.6 | 490 | 888 |
���ͱ���������֮���۵�����ԭ��______________________________��
(5)��������һ�ְ뵼����ϡ������ṹ�ɿ������ʯ�����ڲ���̼ԭ�ӱ�Nԭ�Ӵ��棬��������ĵ�̼ԭ�ӱ�Gaԭ�Ӵ��档
����ͬһ��Gaԭ��������Nԭ�ӹ��ɵĿռ乹��Ϊ__________��
���Ծ�������Ϊ��λ���Ƚ���������ϵ���Ա�ʾ�����и�ԭ�ӵ�λ�ã�����ԭ�ӷ������ꡣ����y��ͶӰ�ľ���������ԭ�ӵķֲ�ͼ��ͼ����2��3��4ԭ�ӵķ������겻���ܵ�����________________��
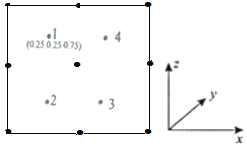
a��(0.75��0.25��0.25) b��(0.25��0.75��0.75)
c��(0.25��0.75��0.25) d��(0.75��0.75��0.75)
�� GaN������N��N��ԭ�Ӻ˼��Ϊa pm��GaNĦ������Ϊ![]() �������ӵ�������ֵΪNA����GaN������ܶ�Ϊ___________
�������ӵ�������ֵΪNA����GaN������ܶ�Ϊ___________![]() ��
��![]() ����
����
���𰸡�![]() N��O��C��Pb��Cs sp2�ӻ� bc Cs2O��PbO�����Ӿ��壬�ۻ����ƻ����Ӽ���CO2�Ƿ��Ӿ��壬�ۻ���˷����»��������»��������Ӽ���������CO2�����۵�ͣ��������Ӿ���PbO�е�Pb2+�뾶С����������������۵�� �������� b
N��O��C��Pb��Cs sp2�ӻ� bc Cs2O��PbO�����Ӿ��壬�ۻ����ƻ����Ӽ���CO2�Ƿ��Ӿ��壬�ۻ���˷����»��������»��������Ӽ���������CO2�����۵�ͣ��������Ӿ���PbO�е�Pb2+�뾶С����������������۵�� �������� b ![]()
��������
(1)Ni��̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2�����Լ۵����Ų�ͼΪ![]() ����Ϊ��
������![]() ��
��
(2)N��2p������������һ�����ܷ���������ԭ�ӣ��ǽ�����Խǿ����һ������Խ������Խǿ����һ������ԽС������C��N��O��Cs��PbԪ�ص�һ�������ɴ�С��˳����N��O��C��Pb��Cs����Ϊ��N��O��C��Pb��Cs��
(3)��ͼ�п��Կ�������������ÿ����ԭ�Ӷ�������3��ԭ���γɹ��ۼ����۵�����Ϊ3���ӻ�����Ϊsp2�ӻ��������ӽṹ�к�����λ������������ѡbc����Ϊ��sp2�ӻ���bc��
(4)�ӱ������ݿ��Կ��������ߵ��۵��ֵ�ϴɴӾ������ͼ���������������з���������������֮���۵�����ԭ��Cs2O��PbO�����Ӿ��壬�ۻ����ƻ����Ӽ���CO2�Ƿ��Ӿ��壬�ۻ���˷����»��������»��������Ӽ���������CO2�����۵�ͣ��������Ӿ���PbO�е�Pb2+�뾶С����������������۵�ߡ���Ϊ��Cs2O��PbO�����Ӿ��壬�ۻ����ƻ����Ӽ���CO2�Ƿ��Ӿ��壬�ۻ���˷����»��������»��������Ӽ���������CO2�����۵�ͣ��������Ӿ���PbO�е�Pb2+�뾶С����������������۵�ߣ�
(5)����ͬһ��Gaԭ��������Nԭ�ӣ���4������ľ�����ȣ����ɵĿռ乹��Ϊ�������塣��Ϊ���������壻
�ڸ���1ԭ�ӵķ��������֪2��3��4ԭ�ӵķ�������ֱ�Ϊ(0.25��0.75��0.25)��(0.75��0.25��0.25)��(0.75��0.75��0.75)����ѡb����Ϊ��b��
�� �辧������Ϊx����GaN������N��N��ԭ�Ӻ˼��Ϊ���϶Խ��߳��ȵ�һ�룬��![]() =a pm��x=
=a pm��x=![]() a pm=
a pm=![]() a ��10-10cm���ھ����У�����4����GaN������GaN������ܶ�Ϊ
a ��10-10cm���ھ����У�����4����GaN������GaN������ܶ�Ϊ![]() =
=![]()
![]() ������
������![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����(Ge)�ǵ��͵İ뵼��Ԫ�أ��ڵ��ӡ����ϵ�����Ӧ�ù㷺���ش��������⣺
(1)Ge�����ڱ��е�λ��__________����̬Geԭ�ӵĺ�������Ų�ʽΪ[Ar] _______���� __________��δ�ɶԵ��ӡ�
(2)�����ԭCO2�Ʊ�CH4��Ӧ�У���״����Zn2GeO4�Ǹ÷�Ӧ�����ô�����Ge��O��Ԫ�ص縺���ɴ���С��˳���� _________��
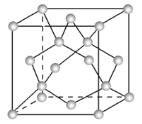
(3)Ge�������н��ʯ�ͽṹ������Geԭ�ӵ��ӻ���ʽΪ ________����֮����ڵ���������___________��
(4)�������������������Ĵ�С����״����֪Ge�����ľ�������a=565.76 pm(1pm=10-12m)�����ܶ�Ϊ_______g��cm��3(�г�����ʽ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������ѧ������ѧ�������о�����Ҫ���塣��Mo��2��C60���ӡ�2��p�����ᶡ����़�2��CO����������λ������װ�ij����ӽṹ��ͼ��ʾ��
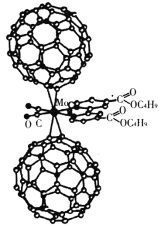
(1)Mo���ڵ������ڵ�VIB�壬��������Ų���Cr���ƣ����Ļ�̬�۵����Ų�ʽ��________������δ�ɶԵ�������________����
(2)�ó�����������CO�ṩ�µ��ӶԵ�ԭ����________(��Ԫ�ط���)��p�����ᶡ�����������Cԭ�ӵ��ӻ���ʽ��________��(��֪��िɿ����������е�һ��CHԭ���ű�Nȡ���Ļ�����)
(3)��֪��C60�����д���̼̼����˫����C60������ÿ��̼ԭ��ֻ�����ڵ�3��̼ԭ���γɻ�ѧ����C60����ֻ��������κ������Σ�������Ķ�����V������F�������E��ѭŷ��������V+F��E=2����һ��C60���ӵĽṹ����_____������κ�____����������ɵ����塣�����ּ���C60��F2��һ�������·�Ӧ���õ����ʵ���ɣ�__________________��
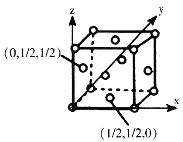
(4)��֪��ij�����и�ԭ�ӵ����λ�ÿ�����ͼ��ʾ��ԭ�������ʾ���������ж����ԭ�����������Ϊ(0��0��0)����(Mo)��һ��������ϵ�ľ���ṹ�У�ÿ��������2��Moԭ�ӣ�����Moԭ��������(0��0��0)��(![]() ��
��![]() ��
��![]() )������������Ϣ���ƶϸþ����ԭ�Ӷѻ���ʽ��_____________����֪�þ�����ܶ�����g��cm-3��Mo��Ħ��������Mg��mol-1�������ӵ�������NA�������о��������Moԭ�Ӻ�֮��ľ���Ϊ_____pm��
)������������Ϣ���ƶϸþ����ԭ�Ӷѻ���ʽ��_____________����֪�þ�����ܶ�����g��cm-3��Mo��Ħ��������Mg��mol-1�������ӵ�������NA�������о��������Moԭ�Ӻ�֮��ľ���Ϊ_____pm��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ȾҪ���������졢��ˮ������������ս��ij�������ۺϴ�����NH4+��ˮ��ҵ��������Ҫ��N2��CO2��SO2��NO��CO�������������ɷ֣���������������̣�
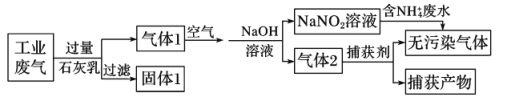
����˵����ȷ���ǣ� ��
A.����1�ijɷ���CaCO3��CaSO3
B.������1��ͨ��Ŀ�����Ҫ����
C.������������������Ҫ��CO
D.������NH4+��ˮʱ�������ķ�ӦΪNH4++5NO2-+4H+��6NO��+4H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ�����������Ũ���ᷴӦ�ĸĽ�װ�ã��������������м��飬ʵ��װ����ͼ��ʾ�����н�������ȷ���ǣ� ��
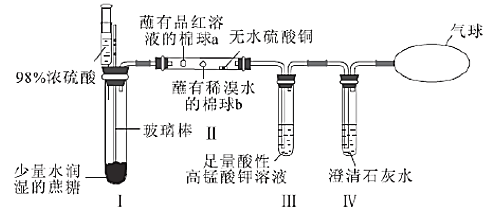
ѡ�� | ���� | ���� |
A. | ����ע��Ũ����ɹ۲쵽�Թ��а�ɫ�����Ϊ��ɫ | ������Ũ�������ˮ�� |
B. | ���й۲쵽����a��b����ɫ | ��������SO2��Ư���� |
C. | ������ˮ����ͭ���� | ˵����Ӧ��������H2O |
D. | ������Һ��ɫ��dz�����г���ʯ��ˮ����� | ˵����CO2���� |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪H2O2�ڴ��������·ֽ����ʼӿ죬�������淴Ӧ���̵ı仯����ͼ��ʾ������˵����ȷ���ǣ� ��
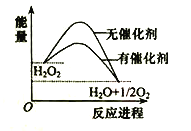
A. �����������С�˷�Ӧ����ЧӦ
B. ��������������H2O2��ƽ��ת����
C. H2O2�ֽ���Ȼ�ѧ����ʽ��H2O2 �� H2O + O2 + Q
D. ��Ӧ��������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��H2S��SO2��Ի��������彡�����������Σ������ҵ�ϲ�ȡ���ַ���������Щ�к�������ŷţ��ش����з����е����⡣
����H2S�ij�ȥ
����1��������H2S��ԭ��Ϊ��H2S+Fe2(SO4)3 =S��+2FeSO4+H2SO4
4FeSO4+ O2+2H2SO4![]() 2Fe2(SO4)3 +2H2O
2Fe2(SO4)3 +2H2O
��1����˾�����ʱ��FeSO4����������������ʱ��5��105�����þ���������______________��
��2����ͼ3��ͼ4�ж�ʹ����˾����������Ϊ______________������Ӧ�¶ȹ��ߣ���Ӧ�����½�����ԭ����______________��
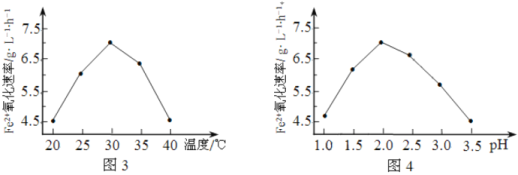
����2����һ�������£���H2O2����H2S
��3�����Ųμӷ�Ӧ��n(H2O2)/n(H2S)�仯���������ﲻͬ����n(H2O2)/n(H2S)=4ʱ����������ķ���ʽΪ__________��
����SO2�ij�ȥ
����1��˫����������NaOH����SO2������CaOʹNaOH����
NaOH��Һ![]() Na2SO3��Һ
Na2SO3��Һ
��4��д�����̢ٵ����ӷ���ʽ��____________________________��CaO��ˮ�д�������ת����
CaO(s)+ H2O (l) =Ca(OH)2(s)![]() Ca2+(aq)+2OH(aq)
Ca2+(aq)+2OH(aq)
��ƽ���ƶ��ĽǶȣ��������̢�NaOH������ԭ��______________________��
����2���ð�ˮ��ȥSO2
��5����֪25����NH3��H2O��Kb=1.8��105��H2SO3��Ka1=1.3��102��Ka2=6.2��108������ˮ��Ũ��Ϊ2.0 mol��L1����Һ�е�c(OH)=_______ mol��L1����SO2ͨ��ð�ˮ�У���c(OH)����1.0��107 mol��L1ʱ����Һ�е�c(![]() )/c(
)/c(![]() )=_____________��
)=_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����2CH3CHO��O2![]() 2CH3COOH������AΪ��Ҫԭ�Ϻϳɻ�����E����ϳ�·����ͼ1��ʾ���ش��������⣺
2CH3COOH������AΪ��Ҫԭ�Ϻϳɻ�����E����ϳ�·����ͼ1��ʾ���ش��������⣺

��1��д���������ʵĹ��������ƣ�B��____________________��D��____________________��
��2����Ӧ�ܵĻ�ѧ����ʽΪ________________________________________________����Ӧ���ͣ�________��
��3��ijѧϰС���������B��������ʵ��װ�����£�����ͼ2װ�ûش����⡣

��װ�ü���ƿ��ʢ�ŵĹ���ҩƷ����Ϊ________(����ĸ)��
A��Na2O2 B��KClC��Na2CO3 D��MnO2
��ʵ������У���װ��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽΪ_______________________________��
������B�Ĵ����������������Ǿ�����ͬ��������Ӧ�������õ���������μӵ�����������ͭ����Һ�м��ȣ�����Ϊ______________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com