
| A�� | 0.2mol•L-1 H2C2O4��Һ��C��H+����C��H2C2O4����C��H2C2O4-����C ��C2O42-�� | |
| B�� | �����£�pH=11��NaOH��Һ��pH=3�Ĵ�����Һ�������Ϻ�������Һ��PH��7 | |
| C�� | �ڣ�NH4��2SO4��Һ�У�C��NH4+��+C��NH3•H2O��=$\frac{1}{2}$C��SO42-�� | |
| D�� | �����ᣨHN3������������ƣ�0.1 mol•L-1NaN3��Һ��C ��N3-����C��Na+����C��OH-����C ��H+�� |
���� A��H2C2O4�Ƕ�Ԫ�����Ե�һ������Ϊ�����ɴ˷������
B����������������Ƶķ�Ӧ�ǰ������ʵ���1��1���еģ��������������Һ�����Դ����ǹ������д�������ʣ�࣬������Һ�����ԣ�
C�����������غ�������
D��NaN3ˮ��Һ�ʼ��ԣ�N3-ˮ�⣮
��� �⣺A��H2C2O4�Ƕ�Ԫ�����Ե�һ������Ϊ����������������������ӣ������뼫��������������Ũ�ȴ�СΪ��C��H2C2O4����C��H+����C��H2C2O4-����C ��C2O42-������A����
B����������������Ƶķ�Ӧ�ǰ������ʵ���1��1���еģ��������������Һ�����Դ����ǹ������д�������ʣ�࣬������Һ�����ԣ�����pH=11��NaOH��Һ��pH=3�Ĵ�����Һ�������Ϻ�������Һ��PH��7����B��ȷ��
C��笠�������������Ҫˮ�⣬��������Һ����������ʽ���ڣ����������غ��֪��C��NH4+��+C��NH3•H2O��=2C��SO42-������C����
D�������ᣨHN3��������������ƣ���������Һ�ʼ��ԣ���NaN3ˮ��Һ�ʼ��ԣ�����C��Na+����C��N3-����C��OH-����C��H+������D����
��ѡB��
���� ���⿼������Ũ�ȵĴ�С�Ƚϣ���Ŀ�Ѷ��еȣ�ע����������Ϣ����Ϊ������Ĺؼ������й��غ��ڽ����е�Ӧ�ã�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��һ����˹�������ܹ���ú������еļ���ﵽһ��Ũ��ʱ��ͨ����������ʾ����������˹�����ǹ���ԭ������ȼ�ϵ�صĹ���ԭ������װ����ͼ��ʾ�����еĹ���������Y2O3-Na2O��O2-���������������ƶ��������й�������ȷ���ǣ�������
��һ����˹�������ܹ���ú������еļ���ﵽһ��Ũ��ʱ��ͨ����������ʾ����������˹�����ǹ���ԭ������ȼ�ϵ�صĹ���ԭ������װ����ͼ��ʾ�����еĹ���������Y2O3-Na2O��O2-���������������ƶ��������й�������ȷ���ǣ�������| A�� | �缫a���������缫��ӦʽΪCH4+4O2--8e-=CO2+2H2O | |
| B�� | �缫b��������O2-�ɵ缫b����缫a | |
| C�� | ��˹�����ǹ���ʱ������ڵ�·�е����ɵ缫a����缫b | |
| D�� | ����·����0.4mol����ת��ʱ��a����2.24L�����������ŵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
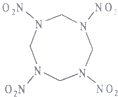 ���ܲ���HMX���ṹ��ͼ��ʾ�������������ij���װҩ�������ƽ�������֣���ըʱ����������������̼��ˮ������˵����ȷ���ǣ�������
���ܲ���HMX���ṹ��ͼ��ʾ�������������ij���װҩ�������ƽ�������֣���ըʱ����������������̼��ˮ������˵����ȷ���ǣ�������| A�� | HMX����ʽΪC4N8O8 | |
| B�� | HMX�У�����C��Nԭ�ӹ�ƽ�� | |
| C�� | ��ը��Ӧ�ǣ�N2���ǻ�ԭ���������������� | |
| D�� | 29.6gHMX��ȫȼ�ղ���������̼8.96L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
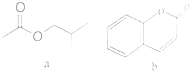
| A�� | a���ڲ������� | |
| B�� | a��b���еĹ�����������ͬ | |
| C�� | 1molb����ܺ�4molH2��Ӧ | |
| D�� | �����ʵ�����a��b�������NaOH������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
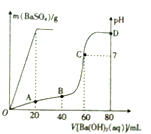 �����£���80mL�������������ɵĻ����Һ�м���0.4 mol•L-1��Ba��OH��2��Һ������BaSO4��������������ҺpH�����Ba��OH��2��Һ������Ĺ�ϵ��ͼ��ʾ��
�����£���80mL�������������ɵĻ����Һ�м���0.4 mol•L-1��Ba��OH��2��Һ������BaSO4��������������ҺpH�����Ba��OH��2��Һ������Ĺ�ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ��ɳ�и�����ѧ�ڵ�13���ܲ������ۺϻ�ѧ�Ծ� ���ͣ�ʵ����
���������յõ�����������Ҫ�ɷ�ΪFeO��Fe3O4��Fe2O3�ȡ����ø������Ʊ��������Ʒ���ȼطʵ��������£�
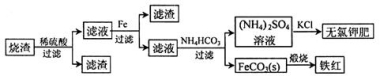
�ش��������⣺
��1������������������Fe2O3��SO2�������ɱ�״����11.2L SO2���壬ת�Ƶ��ӵ����ʵ���Ϊ____________��
��2���������м������۵�������____________�������ӷ���ʽ��ʾ����
��3����֪�����ε��ܽ�����¶ȱ仯��������ͼ��ʾ����ش��������⣺
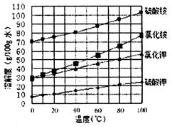
������ҺII�м���NH4HCO3��Һ��������Ӧ�����ӷ���ʽ��________________��
����(NH4)2SO4��Һ����KCl��õ����ȼط�Ӧ���еIJ���Ϊ_________��________��ϴ�ӡ�����ȣ���Ӧ�Ļ�ѧ����ʽΪ_______________��
��4���ú���������������������������ͭ��Һ�Ʊ�CuSO4��5H2O���������£�
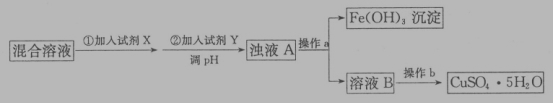
�����Լ�X��Ŀ����_____��������ҺpH���Լ�Y������_____������ĸ��ţ���
a��NaOH
b��CuO
c��NH3��H2O
d��Cu��OH��2CO3
Cu2+Ϊ0.2mol��L-1����Һ��������Fe3+��������Ũ��С��1*10-5mol��L-1ʱ�������ѳ�����ȫ����������ҺpH�ľ�ȷ��Χ��_____����֪��Kap��Cu��OH��2��=2��10-20��Kap��Fe��OH��3��=8.0��10-38��lg2=0.3����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
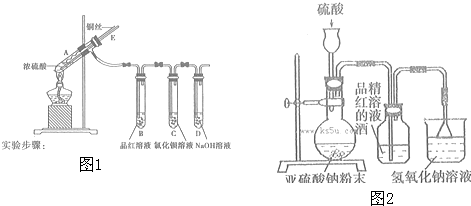
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com