
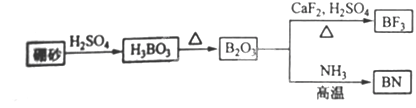
 2BF3����3CaSO4��3H2O
2BF3����3CaSO4��3H2O 2BN��2H2O
2BN��2H2O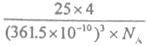 ��2�֣�
��2�֣�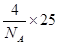 ������������ǣ�361.5��10��3��3���������ܶ���
������������ǣ�361.5��10��3��3���������ܶ���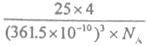 ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ʯ����״�ṹ�У��Թ��ۼ��γɵ���С̼������6��̼ԭ�� |
| B���Ȼ�菉����У�ÿ��Cs+��Χ����8��Cl�� |
| C���Ȼ��ƾ����У�ÿ��Na+��Χ�����������ȵ�Na������6�� |
| D��ʯī�����У�ÿһ����̼ԭ������̼̼����֮��Ϊ2�s3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
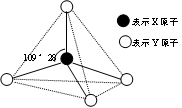
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ԭ�ӵĺ�������ڽ��������ж������ɵ��� |
| B��þ�ͺ�ͭ�͵�ԭ�Ӷѻ���ʽ�ռ���������� |
| C������ԭ���ڻ�ѧ�仯��ʧȥ�ĵ�����Խ�࣬�仹ԭ��Խǿ |
| D���¶����ߣ������ĵ����Խ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Ӿ�����һ�����ڹ��ۼ� |
| B���ڢ�A���⻯������Է����������ӣ��۷е������ߣ�HF�е���� |
| C�����Ӿ������ۻ�ʱ�����Ӽ����ƻ��������Ӿ����ۻ�ʱ����ѧ�������ƻ� |
| D������״̬�µ���ľ���һ���ǽ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| A����λ�� | B�������� | C�����Թ��ۼ� | D���Ǽ��Թ��ۼ� E�����Ӽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ԫ�غ�����ɵľ������е���ԭ�Ӿ��� |
| B���Ѹ�����������ֻ��BCl3��CO2�Ƿ��Ӿ��� |
| C��ͬ��Ԫ�ص�����������γɲ�ͬ���͵ľ��� |
| D����ͬ��Ԫ�ص�����������γ���ͬ���͵ľ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com