
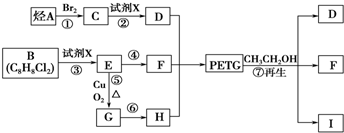
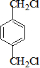
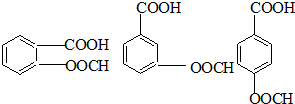
PETGŹĒŅ»ÖÖŠĀŠĶ²ÄĮĻ£¬æÉ»ŲŹÕĄūÓĆ£¬¶Ō»·¾³²»¹¹³ÉČĪŗĪĶžŠ²£¬Ęä½į¹¹¼ņŹ½ČēĻĀ£ŗ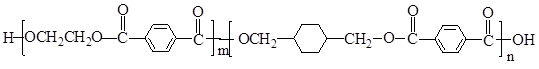
ŅŃÖŖ£ŗRCOOR1£«R2OHØD”śRCOOR2£«R1OH(R”¢R1”¢R2±ķŹ¾Ģž»ł)”£²ÉÓĆČēĻĀĶ¼ĖłŹ¾µÄŗĻ³ÉĀ·ĻßæÉŗĻ³ÉPETG£ŗ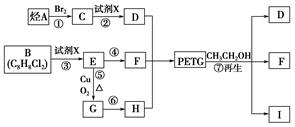
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÉĻŹöø÷²½·“Ó¦ÖŠ£¬ŹōÓŚČ”“ś·“Ó¦µÄÓŠ__________(ĢīŠ“±ąŗÅ)”£
£Ø2£©Š“³ö½į¹¹¼ņŹ½£ŗB_____________________________”¢I_________________________”£
£Ø3£©Š“³ö»Æѧ·½³ĢŹ½£ŗ
·“Ó¦¢Ü ”¢
·“Ó¦¢Ż ”£
£Ø4£©ŗĻ³ÉŹ±Ó¦æŲÖʵĵ„ĢåµÄĪļÖŹµÄĮæn (D)”Ćn (F)”Ćn (H)£½______________(ÓĆm”¢n±ķŹ¾)”£
£Ø1£©¢Ś¢Ū¢ß
£Ø2£©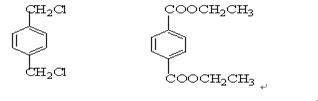
£Ø4£©m £ŗn £ŗ£Øm+n)
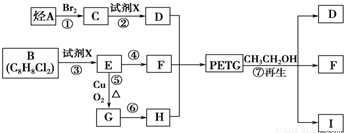
½āĪöŹŌĢā·ÖĪö£ŗÓÉĢāæÉŅŌĶʶĻ³ö£¬AĪŖCH2=CH2£¬BĪŖ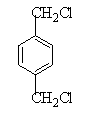 £¬CĪŖCH2BrCH2Br£¬DĪŖHOCH2CH2OH£¬EĪŖ
£¬CĪŖCH2BrCH2Br£¬DĪŖHOCH2CH2OH£¬EĪŖ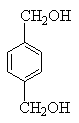 £¬FĪŖ
£¬FĪŖ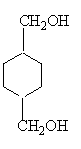 £¬GĪŖ
£¬GĪŖ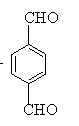 £¬HĪŖ
£¬HĪŖ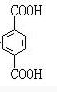
IĪŖ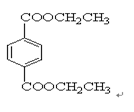 £¬¹Ź£Ø1£©2”¢3”¢7ŹōÓŚČ”“ś·“Ó¦£¬1”¢4ĪŖ¼Ó³É·“Ó¦£¬ĘäĖūĪŖŃõ»Æ·“Ó¦£¬£Ø2£©ÖŠ£¬BIµÄ½į¹¹·Ö±šĪŖ
£¬¹Ź£Ø1£©2”¢3”¢7ŹōÓŚČ”“ś·“Ó¦£¬1”¢4ĪŖ¼Ó³É·“Ó¦£¬ĘäĖūĪŖŃõ»Æ·“Ó¦£¬£Ø2£©ÖŠ£¬BIµÄ½į¹¹·Ö±šĪŖ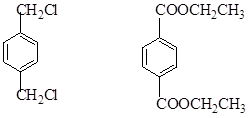 £¬£Ø3£©ÖŠ·“Ó¦¢Ü¢Ż·Ö±šĪŖ£ŗ
£¬£Ø3£©ÖŠ·“Ó¦¢Ü¢Ż·Ö±šĪŖ£ŗ
£Ø4£©ŗĻ³ÉŹ±Ó¦æŲÖʵĵ„ĢåµÄĪļÖŹµÄĮæn (D)”Ćn (F)”Ćn (H)£½m £ŗn £ŗ£Øm+n)”£
æ¼µć£ŗÓŠ»śĶʶĻĢā
µćĘĄ£ŗøĆĢāŹĒŅ»µĄÓŠ»śĶʶĻĢā£¬ŹĒøßæ¼æ¼²éµÄÖŲµć”£ĢāÖŠøų³öµÄŠÅĻ¢Įæ½Ļ“ó£¬ÓŠŅ»¶ØµÄ×ŪŗĻŠŌ£¬ĒŅÓŠŅ»¶ØµÄÄѶȔ£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ





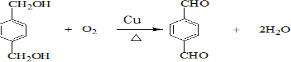
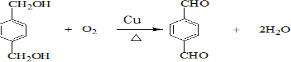

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģɽĪ÷Ź”ø߶ž3ŌĀŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĶʶĻĢā
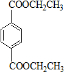
PETGŹĒŅ»ÖÖŠĀŠĶ²ÄĮĻ£¬æÉ»ŲŹÕĄūÓĆ£¬¶Ō»·¾³²»¹¹³ÉČĪŗĪĶžŠ²£¬Ęä½į¹¹¼ņŹ½ČēĻĀ£ŗ
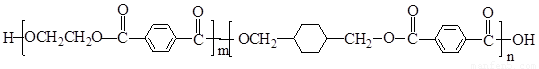
ŅŃÖŖ£ŗRCOOR1£«R2OHØD”śRCOOR2£«R1OH(R”¢R1”¢R2±ķŹ¾Ģž»ł)”£²ÉÓĆČēĻĀĶ¼ĖłŹ¾µÄŗĻ³ÉĀ·ĻßæÉŗĻ³ÉPETG£ŗ
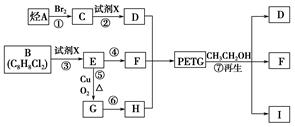
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÉĻŹöø÷²½·“Ó¦ÖŠ£¬ŹōÓŚČ”“ś·“Ó¦µÄÓŠ__________(ĢīŠ“±ąŗÅ)”£
£Ø2£©Š“³ö½į¹¹¼ņŹ½£ŗB_____________________________”¢I_________________________”£
£Ø3£©Š“³ö»Æѧ·½³ĢŹ½£ŗ
·“Ó¦¢Ü ”¢
·“Ó¦¢Ż ”£
£Ø4£©ŗĻ³ÉŹ±Ó¦æŲÖʵĵ„ĢåµÄĪļÖŹµÄĮæn (D)”Ćn (F)”Ćn (H)£½______________(ÓĆm”¢n±ķŹ¾)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
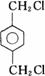
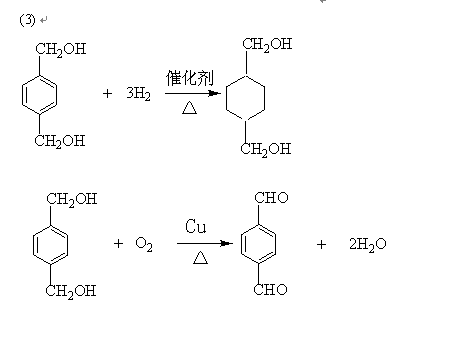
ÕāÖÖ²ÄĮĻæɲÉÓĆČēĻĀĶ¼ĖłŹ¾µÄĀ·ĻßŗĻ³É£ŗ
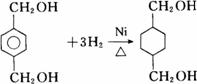
ŅŃÖŖ£ŗ
¢ŁnHOOC”ŖCOOH+nHO”ŖCH2”ŖCH2”ŖOH![]()
![]() +2nH2O
+2nH2O
¢ŚRCOOR1+R2OH![]() RCOOR2+R1OH(R1”¢R2”¢R±ķŹ¾Ģž»ł)£¬ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
RCOOR2+R1OH(R1”¢R2”¢R±ķŹ¾Ģž»ł)£¬ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ÉĻŹöø÷²½·“Ó¦ÖŠ£¬ŹōÓŚČ”“ś·“Ó¦µÄÓŠ____________(ĢīŠ“±ąŗÅ)”£
(2)Š“³ö¢ńµÄ·Ö×ÓŹ½__________£¬BµÄ½į¹¹¼ņŹ½___________”£
(3)Š“³ö»Æѧ·½³ĢŹ½£ŗ·“Ó¦¢Ü_____________________£»¢Ż_____________________”£
(4)ŗĻ³ÉŹ±Ó¦æŲÖʵĵ„ĢåµÄĪļÖŹµÄĮæn(D)£ŗn(F)£ŗn(H)__________(ÓĆm”¢n±ķŹ¾)”£
(5)ÓėE»„ĪŖĶ¬·ÖŅģ¹¹Ģ壬ŹōÓŚ·ÓĄąĒŅ±½»·ÉĻÖ»ÓŠĮ½øö»„ĪŖ¶ŌĪ»Č”“ś»łµÄ»ÆŗĻĪļÓŠ____________ÖÖ£¬Ęä½į¹¹¼ņŹ½·Ö±šĪŖ_____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012Äź±±¾©ŹŠ¶«³ĒĒųøßæ¼»ÆѧŅ»Ä£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
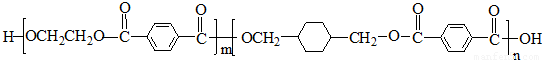
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com