

(1)��������ʢ_______��Һ����������ʢ_______��Һ��������ʢ_______��Һ��
(2)ʵ��ʱ�ر�_______ (a��b)����_______ (a��b)��������Ӧ�Ļ�ѧ����ʽ��_______�������õ���������_______��
(3)��������һ������ر�_______ (a��b)����_______ (a��b)��������Ӧ�Ļ�ѧ����ʽ��______________�������õ���������_______��
 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ҫ����CO��CO2���ֱ�õ���������壬ijѧ���������ͼװ�ã�����a�����У�b�Ƿ�Һ©������������

(1)��������ʢ??????????_______��Һ����������ʢ_______��Һ��������ʢ_______��Һ��
(2)ʵ��ʱ�ر�_______ (a��b)����_______ (a��b)��������Ӧ�Ļ�ѧ����ʽ��_______�������õ���������_______��
(3)��������һ������ر�_______ (a��b)����_______ (a��b)��������Ӧ�Ļ�ѧ����ʽ��______________�������õ���������_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������������һ�и߶���ѧ�����п������ƻ�ѧ�Ծ����������� ���ͣ������
��12�֣� �ش��������⣺
��1����ҵ�ϳɰ���ԭ���ǵ����������������Ǵӿ����з�������ģ�ͨ��ʹ�õ����ַ��뷽���������������������� �ͽ�������̼��Ӧ���ȥCO 2����������Դ����ˮ��̼�⻯���ﷴӦ������CO, д��������Ȼ��Ϊԭ����ȡ�����Ļ�ѧ��Ӧ����ʽ���������������������������� ������������������������������������������������
��2���豸A�к��е����������ú���Ƚ��������豸A�з����Ļ�ѧ��Ӧ����ʽΪ��������������������������������������������
��3�� �豸B�������������������� ������m��n������ͨˮ�ڣ���ˮ������������ ���m����n������
��4���豸C�������������������������������������������� ��
��5����ԭ�����Ʊ������л���CO�Դ����ж������ã�����ȥԭ�����е�CO����ͨ�����·�Ӧ��ʵ�֣�CO(g)+H2O(g) ��CO2 (g)+ H2 (g)����֪1000Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.627����ҪʹCO��ת������90%������ʼ����c(H2O)��c(CO)����������������
��CO2 (g)+ H2 (g)����֪1000Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.627����ҪʹCO��ת������90%������ʼ����c(H2O)��c(CO)����������������
��������������λ��Ч���֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�������и߶���ѧ�����п������ƻ�ѧ�Ծ��������棩 ���ͣ������
��12�֣� �ش��������⣺

��1����ҵ�ϳɰ���ԭ���ǵ����������������Ǵӿ����з�������ģ�ͨ��ʹ�õ����ַ��뷽���������������������� �ͽ�������̼��Ӧ���ȥCO 2����������Դ����ˮ��̼�⻯���ﷴӦ������CO, д��������Ȼ��Ϊԭ����ȡ�����Ļ�ѧ��Ӧ����ʽ���������������������������� ������������������������������������������������
��2���豸A�к��е����������ú���Ƚ��������豸A�з����Ļ�ѧ��Ӧ����ʽΪ��������������������������������������������
��3�� �豸B�������������������� ������m��n������ͨˮ�ڣ���ˮ������������ ���m����n������
��4���豸C�������������������������������������������� ��
��5����ԭ�����Ʊ������л���CO�Դ����ж������ã�����ȥԭ�����е�CO����ͨ�����·�Ӧ��ʵ�֣�CO(g)+H2O(g)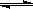 ��CO2 (g)+ H2
(g)����֪1000Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.627����ҪʹCO��ת������90%������ʼ����c(H2O)��c(CO)����������������
��CO2 (g)+ H2
(g)����֪1000Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.627����ҪʹCO��ת������90%������ʼ����c(H2O)��c(CO)����������������
��������������λ��Ч���֣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com