
��ȩ��ҽҩ��Ⱦ�ϡ����ϵ���ҵ�ж����Ź㷺��Ӧ�á�ʵ����ͨ����ͼ��ʾ�������ɼױ������Ʊ�����ȩ���Իش��������⡣
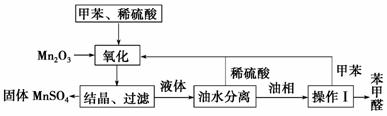
(1)Mn2O3�����ױ��ķ�Ӧ��Ҫ���Ͻ��裬�����������__________________
______________________________________________________��
(2)�ױ���������õ��Ļ����ͨ���ᾧ�����˽��з��룬�ù������轫�������ȴ����Ŀ����________________________________________________
________________________��
(3)ʵ������У���ѭ��ʹ�õ�������________��________��
(4)ʵ���з���ױ��ͱ���ȩ���õIJ�������______����ԭ����__________
______________________________________________________________��
������(1)����Mn2O3��Һ̬�ļױ���Ӧ���Ӵ����С����Ӧ���������������ʹMn2O3�ͼױ���ֽӴ����ӿ췴Ӧ���ʡ�(2)������ͼ���Կ�������Ӧ��Ļ�����к��������̡�����ȩ�ȡ����ᾧ�����ˡ��ɵþ��������̣�������オ����Ϊ�˽��������̵��ܽ�ȣ�ʹ�����Һ�нᾧ������(3)������ͼ�м�ͷ��ָ�����ֱ�ӿ�������ѭ����������ϡ����ͼױ���(4)���Ʊ�ԭ����֪���ױ��ͱ���ȩ����ܽ���л�������Ͳ��к��е�����Ϊ����ȩ��δ�������ļױ���Ҫʹ���Ƿ��룬ֻ��������ķ�����
�𰸡�(1)ʹ��Ӧ���ֽӴ����ӿ췴Ӧ����
(2)����MnSO4���ܽ��
(3)ϡ���ᡡ�ױ�
(4)�������üױ��ͱ���ȩ�ķе����ʹ���߷���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и������ʼ�������ˮ���γ�����Һ��һ��ʱ�����������ᣬ���Dz�����ʧ������ɫ��ζ�������ɵ��ǣ� ��
A��Ba��NO3��2��Na2SO3 B��BaCO3��Na2SO4
C��BaCl2��Na2SO4 D��Ca��HCO3��2��Ca��OH��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����и���������ָ����Һ���ܴ����������(����)
A��pH��1����Һ�У�Fe2����NO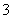 ��SO
��SO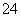 ��Na��
��Na��
B��ˮ�������c(H��)��10��12 mol/L����Һ�У�Ca2����K����Cl����HCO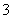
C��c(H��)/c(OH��)��1012��ˮ��Һ�У�NH ��Al3����NO
��Al3����NO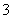 ��Cl��
��Cl��
D��c(Fe3��)��0.1 mol/L����Һ�У�K����ClO����SO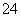 ��SCN��
��SCN��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ѡ������ʵ�鷽�������ᴿ���ʣ��������ᴿ������������ں����ϡ�
A����ȡ��Һ��B��������C���ؽᾧ��D����Һ��E������F�����ˡ�G��ϴ��
(1)________���뱥��ʳ��ˮ��ɳ�ӵĻ���
(2)______������غ��Ȼ��ƵĻ����Һ�л������ء�
(3)________����ˮ�����͵Ļ���
(4)________����CCl4(�е�Ϊ76.75 ��)�ͼױ�(�е�Ϊ110.6 ��)�Ļ���
(5)________��ȥ���������е���ϩ��
(6)________��ȡ��ˮ�еĵ⡣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ���ᴿ�±���������(������Ϊ����)���йس����Լ��ͷ��뷽����ѡ�����ȷ����
(����)
| ѡ�� | ���ᴿ������ | �����Լ� | ���뷽�� |
| A | ����(��ϩ) | ��ˮ | ��Һ |
| B | ������Һ(NaCl) | ˮ | ���� |
| C | CH3CH2OH (CH3COOH) | CaO | ���� |
| D | CO2(SO2) | Na2CO3��Һ | ϴ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Һ������Һ����Һ�ͽ��嶼��(����)
A���ȶ���Һ�� B������Һ��
C������� D��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ѵ��ۺ�NaCl��Һװ���Ĥ���ڣ���������ˮ�н����������Իش�
(1)֤������δ����Ĥ��Cl��������Ĥ��ʵ�鷽����_______________ ___________________________________________________________________________��
(2)֤��������NaCl��Һ�ѷ�����ȫ��ʵ�鷽����___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ݻ�������ܱ������д������·�Ӧ��2SO2(g)��O2(g)  2SO3(g) ��H<0��ij�о�С���о���������������ʱ���ı�ijһ������������Ӧ��Ӱ�죬���з�����ȷ���� ( )
2SO3(g) ��H<0��ij�о�С���о���������������ʱ���ı�ijһ������������Ӧ��Ӱ�죬���з�����ȷ���� ( )
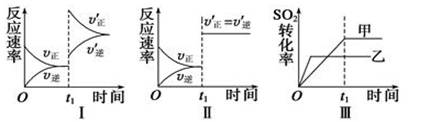
A��ͼ���ʾ����t1ʱ������O2��Ũ�ȶԷ�Ӧ���ʵ�Ӱ��
B��ͼ���ʾ����t1ʱ�̼��������Է�Ӧ���ʵ�Ӱ��
C��ͼ���ʾ���Ǵ����Ի�ѧƽ���Ӱ�죬�ҼĴ�Ч�ʱ��Ҹ�
D��ͼ���ʾ����ѹǿ�Ի�ѧƽ���Ӱ�죬���ҵ�ѹǿ�ϸ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������������(����)
A�������к���̼������ʴ�����ȴ�����
B���������ӵ��������������Ӵ�������
C��������Ʒ�϶�ͭʱ���Ƽ�Ϊ������ͭ��Ϊ���Һ
D����������Ƕͭ�飬���ܲ��ױ���ʴ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com