
����Ŀ�����Ȼ���(SnCl4)�Ǻϳ��л����������ԭ�ϣ����۵�Ϊ��33�棬�е�Ϊ114�棬�ڳ�ʪ�Ŀ�����ǿ��ˮ������д̼��Եİ�ɫ����������֮һΪSnO2��ʵ�����Ʊ����Ȼ����IJ����ǣ������������ڣ�Ȼ��������ˮ���Ƴ���������������������뷴Ӧ���У�����Ӧ���л�����ͨ������������(װ��C�е��Լ���δ����)
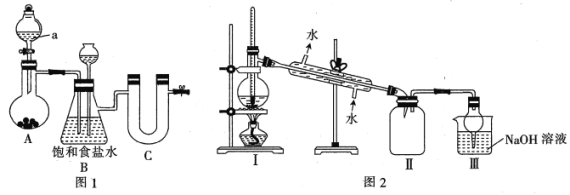
I.ͼ1Ϊʵ�����Ʊ�����������ʵ��װ��(�г�װ������)
(1)��װ���Ʊ�����ѡ�õ�ҩƷΪƯ�۾�����[��Ҫ�ɷ�Ca(ClO2]��Ũ���ᣬA�з�����Ӧ�Ļ�ѧ����ʽΪ_________________________________��
(2)װ��B�еı���ʳ��ˮ�ܹ���ȥCl2�е�HCl�������___________���á�
(3)�Լ�������Ϊ___________(����)��
A.��ˮ�Ȼ��� B.Ũ���� C.���������� D.��ʯ��
(4)����������������Ŀ����_________________________________��
(5)���Ȼ����ڳ�ʪ������ˮ��Ļ�ѧ����ʽΪ_________________________________��
��.ͼ2������SnCl4��װ��
(6)��װ��������������֮�������Ƿֱ���____________________��______________________��
(7)ʵ���õ������к��н���ͭ��ijͬѧ�������ʵ��ⶨ�����Ĵ��ȣ�
��һ������ȡ7.500g������������ϡ�����У���ַ�Ӧ����ˣ�
�ڶ���������Һ�м������Fe2(SO4)3�������ɵ�Sn2+������Sn4+��
����������0.9500mol��L��1��K2Cr2O7��Һ�ζ����ɵ�Fe2+��������Ӧ�ķ���ʽΪFe2++Cr2O72��+H+��Cr3++Fe3++H2O(δ��ƽ)
���ﵽ�ζ��յ�ʱ������21.00mLK2Cr2O7��Һ���Լ������������İٷֺ�����___________(�������4λ��Ч����)��
���𰸡�Ca��ClO��2+4HCl��Ũ��=CaCl2+2Cl2��+2H2O ��ȫƿ AC �����������ĽӴ�������ӿ췴Ӧ���� SnCl4+2H2O=SnO2+4HCl �¶ȼ�ˮ����λ�ò��� ��͢�֮��ȱ�ٸ���װ�ã� 94.96%
��������
(1)ͼIװ���Ʊ�����ѡ�õ�ҩƷΪƯ�۾�����[��Ҫ�ɷ�Ca(ClO2]��Ũ���ᷴӦ�����Ȼ��ơ�������ˮ��A�з�����Ӧ�Ļ�ѧ����ʽΪCa��ClO��2+4HCl��Ũ��=CaCl2+2Cl2��+2H2O���𰸣�Ca��ClO��2+4HCl��Ũ��=CaCl2+2Cl2��+2H2O��
��2���Ʊ��������к���HCl������ʳ��ˮ��ȥCl2�е�HCl����C�з���������װ������ѹ����B�г���©����Һ���������γ�ˮ��������B�����˰�ȫƿ�����á��ʴ�Ϊ����ȫƿ��
(3) ���Ȼ�������ˮ�⣬ͨ�������Ӧ���U�ι����Լ�X��������������Ϊ���Ը���������Ը����������������������ΪҺ���������������岻��ͨ��������ѡ���Ȼ��ơ����������ף��ʴ�Ϊ��AC��
(4)����������������Ŀ���ǣ������������ĽӴ�������ӿ췴Ӧ���ʡ��ʴ�Ϊ�������������ĽӴ�������ӿ췴Ӧ���ʣ�
(5) ���Ȼ�����ˮǿ��ˮ�⣬����ˮ��ԭ����֪��Ӧ����Sn(OH)4��HCl������֮һ�ǹ�̬����������˵��Sn(OH)4�ֽ�����SnO2��H2O����ˮ������SnO2��HCl����Ӧ����ʽΪ��SnCl4+2H2O=SnO2+4HCl���ʴ�Ϊ��SnCl4+2H2O=SnO2+4HCl��
(6)�¶ȼ�ˮ����λ�ò��ԣ�Ӧ��������ƿ֧�ܿڴ������Ȼ�����ˮǿ��ˮ�⣬��װ�â��е�ˮ����������У�ʹ�䷢��ˮ�⣬����֮��ȱ�ٸ���װ�ã��ʴ�Ϊ���¶ȼ�ˮ����λ�ò��ԣ���͢�֮��ȱ�ٸ���װ�ã�
��7���ڶ���������Fe2(SO4)3����Sn2+������Sn4+����������ԭΪ�������ӣ���Ӧ�����ӷ���ʽΪ��2Fe3++Sn2+=2Fe2++Sn4+����Ӧ�����ĵ�K2Cr2O7�����ʵ���Ϊ0.9500mol/L��0.021L=0.01995mol��6Fe2++Cr2O72��+14H+=2Cr3++6Fe3++7H2O�����ݷ���ʽ�ɵù�ϵʽ3Sn��3Sn2+��3Fe2(SO4)3��6Fe3+��K2Cr2O7����n(Sn)=3n(K2Cr2O7)=3��0.01995mol=0.05985mol�� Sn������Ϊ��0.05985mol��119g/mol=7.122g��������Sn�İٷֺ���Ϊ=7.12g/7.500��100%=94.96%����ˣ�������ȷ���ǣ�94.96%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ǵ��������ı���ɡ���������������и�ǿ�������ԡ�ʵ���ҿɽ�����ͨ����ѹ�ŵ������ȡ������3O2![]() 2O3��
2O3��
(1)��������Ӧ����30%������ת��Ϊ�����������û�������ƽ��Ħ������______(����һλС��)��
(2)��8 L����ͨ���ŵ�ָܺ���ԭ״�����õ�����6.5 L�������г��������___________��
(3)���������£�O3��O2������ͭ�۷�Ӧ��ʵ�����н������ͳ����Ļ������0.896 L(��״����)ͨ��ʢ��20.0 gͭ�۵ķ�Ӧ���У���ּ��Ⱥ�����屻��ȫ���գ���ĩ��������Ϊ21.6 g����ԭ��������г������������___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������(FeC2O4��xH2O)Ϊ����ɫ��ĩ��������ˮ������������Ӱ����������ҩ��ҵ��ij��ѧ��ȤС��������ʽ�������̽�����ش��������⣺
I.����̽��
ѡ�������Լ����ʵ�鷽��������±����ݡ�
�Լ�������KMnO4��Һ��K3[Fe(CN)6]��Һ
���� | ���� | ��������� |
(1)ȡ�������������������Թ��У�����2mLˮ������ | �е���ɫ�������ϲ���Һ��ɫ | ��������������ˮ |
(2)��������2mLϡ���ᣬ�� | ___________ | ���������������ᣬ��������ǿ�ڲ��� |
(3)����(2)������Һ�еμӼ���K3[Fe(CN)6]��Һ | ������ɫ���� | ___________ |
(4)��___________ | ��___________ | H2C2O4��C2O42�����л�ԭ�� |
��.����̽�����ζ�ʵ���x��ֵ
(5)�ζ�ǰ�����в�������ȷ˳����___________(����ĸ���)��
a.��0.1000mol/L������KMnO4��Һ��ϴ
b.��©����ϴ
c.�ž��ζ��ܼ�������ݲ�����Һ��
d.ʢװ0.1000mol/L������KMnO4��Һ
e.��ʼ��������¼Ϊ0.50mL
(6)��ȡng��Ʒ����������ϡ�����ܽ⣬�ò���(5)���ı�KMnO4��Һֱ�ӵζ�������жϵζ��յ�?_______________��
(7)�յ����Ϊ20.50mL���������ʵ���������x=___________(�ú�n�Ĵ���ʽ��ʾ��FeC2O4����Է�������Ϊ144)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������ͨ����MnO2��Ũ���ᷴӦ��ȡ�������䷴Ӧ�Ļ�ѧ����ʽΪ��MnO2 + 4HCl(Ũ) ![]() MnCl2 + Cl2��+ 2H2O
MnCl2 + Cl2��+ 2H2O
��1��������������ʾ�÷�Ӧ����ת�Ƶķ������Ŀ��___________��
��2���ڸ÷�Ӧ�У�����1 mol Cl2���ɣ���������HCl�����ʵ�����___________��ת�Ƶ��ӵ���Ŀ��_____________��
��3��ij�¶��£���Cl2ͨ��NaOH��Һ�У���Ӧ�õ�����ClO-��ClO3-���ʵ���֮��Ϊ1��1�Ļ��Һ����Ӧ�Ļ�ѧ����ʽ�� _________________________ ��
��4����ֽ�����˶�����������ϴʱ������ʹ�á�����顱����Ҫ�ɷ������ᣩ�롰84����Һ������Ҫ�ɷ���NaClO�����������ж����¼����Ը�����Ļ�ѧ֪ʶ������ԭ���ǣ������ӷ���ʽ��ʾ��_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾװ�ó�ȥ����CN����Cl����ˮ�е�CN��ʱ��������Һ��pHֵΪ9��10������������ClO����CN������Ϊ��������Ⱦ������������˵������ȷ����
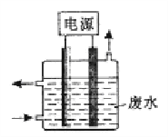
A. ��ʯī����������������
B. �����ĵ缫��ӦʽΪ��Cl����2OH����2e��=== ClO����H2O
C. ��������������ǿ�������������Լ���
D. ��ȥCN���ķ�Ӧ��5ClO����2CN����2H+ === N2����2CO2����5Cl����H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪H2S��һ�ֶ�Ԫ���ᣬ�ش��������⣺
��1��0.1mol/L NaHS��Һ�Լ��ԣ���c(S2��)___________c(H2S)������������ ����С���� ���������� �� ��
��2�������£���0.2 mol/L��H2S��Һ����ε���0.2 mol/L NaOH��Һ�����ԣ���ʱ��Һ��������ʾ��ϵһ����ȷ����________��
A��c(H��)��c(OH��)��1��10��14
B��c(Na��)��c(HS��)��2c(S2��)
C��c(Na��) > c(HS��)��c(S2��)+c(H2S)
D��c(H2S) > c(S2��)
��3����֪�����£�CaS������Һ�д���ƽ�⣺CaS(s)![]() Ca2��(aq)��S2��(aq)����H��0��
Ca2��(aq)��S2��(aq)����H��0��
���¶�����ʱ��Ksp________ (����������������С��������������ͬ)��
���μ�����Ũ���ᣬc(Ca2��)________��ԭ����______________________________________________(�����ֺ����ӷ���ʽ˵��)��
��4������CaS����Һ�м���Cu(NO3)2��Һ������һ�ֺ�ɫ�������ʣ�д���ù����з�Ӧ�����ӷ���ʽ___________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ϳɰ��������ѧ�����ϵ�һ���ش�ͻ�ƣ���Ӧԭ��ΪN2(g)��3H2(g)![]() 2NH3(g) ��H����92.4 kJ��mol��1��һ�ֹ�ҵ�ϳɰ��ļ�ʽ����ͼ���£�
2NH3(g) ��H����92.4 kJ��mol��1��һ�ֹ�ҵ�ϳɰ��ļ�ʽ����ͼ���£�
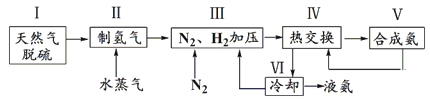
��1���ٲ��������������ԭ�����£�
a��CH4(g)��H2O(g)![]() CO(g)��3H2(g)��K1
CO(g)��3H2(g)��K1
b��CO(g)��H2O(g)![]() CO2(g)��H2(g)��K2
CO2(g)��H2(g)��K2
��ӦCH4(g)��2H2O(g)![]() CO2(g)��4H2(g)��K=_______________(�ú�K1��K2�Ĵ���ʽ��ʾ)��
CO2(g)��4H2(g)��K=_______________(�ú�K1��K2�Ĵ���ʽ��ʾ)��
��T1�¶�ʱ�����ڷ�Ӧ��CO(g)+ H2O(g)![]() CO2(g)+ H2(g)����2 L�ĺ����ܱ�������ͨ��һ������CO��H2O(g)�����ﵽƽ���Ӧ����Q kJ�����������������䣬ֻ����ԭƽ����ϵ����ͨ��0.20 mol H2O(g)��������˵����ȷ����_____________��
CO2(g)+ H2(g)����2 L�ĺ����ܱ�������ͨ��һ������CO��H2O(g)�����ﵽƽ���Ӧ����Q kJ�����������������䣬ֻ����ԭƽ����ϵ����ͨ��0.20 mol H2O(g)��������˵����ȷ����_____________��
A��CO��ת���ʽ����� B���ﵽ��ƽ��ʱ�ķ�Ӧ�Ȧ�H �� ��Q
C��������ܶȽ����� D��H2O�������������
��2����3 molH2��2 molN2����ij���º�ѹ�����У������ϳɰ��ķ�Ӧ��3H2(g) ��N2(g) ![]() 2NH3(g)
2NH3(g)
�ٴ�ƽ��ʱNH3��Ũ��Ϊc mol��L-1�������¶Ȳ��䣬��������ȷֱ�����������ƽ���NH3��Ũ�Ȳ�Ϊc mol��L-1����_________��
A��6 molH2 + 4 molN2
B��0.75 molH2 + 0.75 molN2 + 0.5 molNH3
C��3 molH2+ 1 molN2 + 2 mol NH3
�� �����ں��º��ݵ������з�Ӧ���ﵽƽ��ʱNH3��Ũ��Ϊc1 mol��L-1����c________c1���<����>����=������ԭ����________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͬ��ԭ�����Ķ�������������������ʵ���֮����___������֮����___����4mol/LCuSO4��2mol/LH2SO4��Һ�������ϣ������Ϻ���Һ��������ڻ��ǰ��Һ�����֮�ͣ���������Һ��C(CuSO4)=_____mol/L��C(SO42-)=___mol/L����10����2mol/L��������Һ��ˮϡ�͵�0.5mol/L�������Ϊ____������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���۲����о��������ʵ�һ�ֻ���������һͬѧ��һС�������¶���ڿ����У��۲쵽������������ɫ![]() ��Ұ�
��Ұ�![]() ���ɫ
���ɫ![]() ����Һ��
����Һ��![]() ��ɫ���壬����˵����ȷ���ǣ� ��
��ɫ���壬����˵����ȷ���ǣ� ��
A.�ٷ����˻��Ϸ�ӦB.�ڱ��ɫ����Ϊ������̼����
C.����̼�������տ����е�ˮ�����γ�����ҺD.��ֻ���������仯
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com