
���� ��1��ʵ��ʱӦ�ȳ���һ�������Ĺ��壬�ܽ�����Ƴ���Һ����ȡ����Һ����ƿ�У�Ȼ���ñ�Һ���еζ���
��2���ζ�����ʹ��ǰ��Ҫ����Ƿ�©ˮ��
��3������ƿ�µ�һ�Ű�ֽʹ�ζ��յ���ɫ�仯�����ԣ����ڷֱ棻
��4�����ҺŨ�ȱ�С���������
��5�����ݷ�Ӧ���ĵ���������������Ƶ�������Ȼ��������Ʒ�Ĵ��ȣ�
��� �⣺��1��ʵ��ʱӦ�ȳ���һ�������Ĺ��壬�ܽ�����Ƴ���Һ����ȡ����Һ����ƿ�У�Ȼ���ñ�Һ���еζ������岽��Ϊ��
C������ƽ��ȷ��ȡ�ռ���ƷWg�����ձ��м�����ˮ�ܽ�
A����250mL����ƿ�ж��ݳ�250mL�ռ���Һ
B������Һ����ȡ25mL�ռ���Һ����ƿ�в��μӼ��μ���ָʾ��
D�������ʵ���Ũ��ΪM mol/L�ı�HCl��Һװ����ʽ�ζ��ܣ�����Һ�棬���¿�ʼ�̶�ΪV1mL
E������ƿ�µ�һ�Ű�ֽ���ζ����յ㣬��¼�յ�̶�ΪV2mL��
����ȷ�IJ��������˳��ΪCABDE��
�ʴ�Ϊ��CABE��
��2��Ϊ�˱���ζ���©Һ��Ӱ��ζ�����������к͵ζ�ǰ��Ҫ���ζ����Ƿ�©ˮ��
�ʴ�Ϊ����©��
��3���к͵ζ��У�Ϊ��ȷ�ж��յ�ʱ��ɫ�ı仯�������Ҫ����ƿ�µ�һ�Ű�ֽ��
�ʴ�Ϊ������ȷ�ж��յ�ʱ��ɫ�ı仯�����
��4������ʽ�ζ���δ���������Һ��ϴ����Һ��ϡ�ͣ���Һ��Ũ�ȱ�С���ζ�ʱ���ĵı�Һ���ƫ�ⶨ���ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��5���ε����ĵ�HCl�����ʵ���Ϊ��n��HCl��=cV=��V2-V1����10-3L��Mmol/L�����ݷ�Ӧ���̿�֪��n��NaOH��=n��HCl��=M��V2-V1����10-3mol��ԭ��Ʒ���������Ƶ����ʵ���Ϊ��M��V2-V1����10-3mol��$\frac{250}{25}$=M��V2-V1����10-2mol�����������Ƶ�����Ϊ��m��NaOH��=nM=40M��V2-V1����10-2g��
���Ը��ռ���Ʒ�Ĵ���Ϊ��$\frac{40M��{V}_{2}-{V}_{1}����1{0}^{-2}g}{Wg}$��100%=$\frac{{2M��{V_2}-{V_1}��}}{5W}��100%$��
�ʴ�Ϊ��$\frac{{2M��{V_2}-{V_1}��}}{5W}��100%$��
���� ���⿼���к͵ζ��������ڻ�ѧʵ����������Լ����ʵĺ����IJⶨ�����⣬��Ŀ�Ѷ��еȣ���ȷ�к͵ζ��IJ�������Ϊ���ؼ�������������ѧ���ķ�����������ѧʵ��������


 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
| c��NO������10-4mol•L-1�� | 10.0 | 4.50 | 2.50 | 1.50 | 1.00 | 1.00 |
| C��CO������10-3mol•L-1�� | 3.60 | 3.05 | 2.85 | 2.75 | 2.70 | 2.70 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
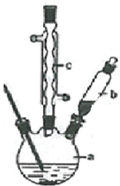 ������ͭ�Ǻϳ��������������в���--��������ͭ����Ҫǰ����֮һ������������һ��ʵ���Һϳ�·�ߣ�
������ͭ�Ǻϳ��������������в���--��������ͭ����Ҫǰ����֮һ������������һ��ʵ���Һϳ�·�ߣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
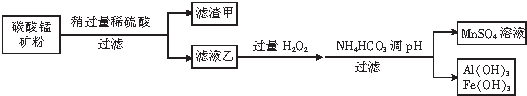
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
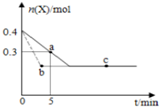 �ں���2L�ܱ�������ͨ������X��������Ӧ��2X��g���TY��g����H��0��X�����ʵ���n��x����ʱ��t�仯��������ͼ��ʾ��ͼ�е������߷ֱ���������������Σ�������������ȷ���ǣ�������
�ں���2L�ܱ�������ͨ������X��������Ӧ��2X��g���TY��g����H��0��X�����ʵ���n��x����ʱ��t�仯��������ͼ��ʾ��ͼ�е������߷ֱ���������������Σ�������������ȷ���ǣ�������| A�� | ʵ�߱�ʾʹ�ô��������� | |
| B�� | b��c���������Ӧ����Ӧ�����´ﵽ������� | |
| C�� | ��Ӧ�ӿ�ʼ��a���ƽ����Ӧ���ʿɱ�ʾΪv��Y��=0.01mol/��L•min�� | |
| D�� | ��Ӧ���е�a��ʱ�ų����������ڷ�Ӧ���е�b��ʱ�ų������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 5min����O2��ʾ�ķ�Ӧ����Ϊ0.12mol/��L•min�� | |
| B�� | �����������ʹ�÷�Ӧ�Ļ�ѧ��Ӧ���ʼӿ죬��H��С | |
| C�� | SO2��ƽ��Ũ��Ϊ0.12mol/L | |
| D�� | �ﵽƽ��ʱ���������������������÷�Ӧ�Ļ�ѧ��Ӧ���ʼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH4Cl��s���TNH3��g��+HCl��g�������²����Է����У�˵���÷�Ӧ�ġ�H��0 | |
| B�� | ��п����Ʒ�Ʋ����������Ʒ������ǰ���������⣬�����������෴ | |
| C�� | ����N2��g��+3H2��g��?2NH3��g������������������ʱ��ѹ���������ʹѹǿ��������Ӧ���淴Ӧ�����Լ�H2��ƽ��ת���ʾ����� | |
| D�� | 25��ʱNH3•H2Oϡ��Һ�У���ˮϡ��$\frac{c��{H}^{+}��•c��N{H}_{3}•{H}_{2}O��}{c��N{H}_{4}^{+}��}$��ֵ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

| �¶�/�� | 20 | 40 | 60 | 80 |
| KIO3g/100gˮ | 8.08 | 12.6 | 18.3 | 24.8 |
| �������� | ʵ������ |
| ȡ1g������NaCl����3mLˮ�����Һ | ��Һ�ޱ仯 |
| ����5�ε�����Һ��1mL0.1mol•L-1KI��Һ���� | ��Һ�ޱ仯 |
| Ȼ���ٵ���1mol•L-1��H2SO4������� | ��Һ����ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C2H5OH��l��+3O2��g���T2CO2��g��+3H2O��g������H=-1367.0 kJ/mol��ȼ���ȣ� | |
| B�� | NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l������H=-57.3kJ/mol���к��ȣ� | |
| C�� | S��s��+O2��g���TSO2��g������H=+269.8kJ/mol����Ӧ�ȣ� | |
| D�� | 2HCl��g���TCl2��g��+H2��g������H=-184.6kJ/mol����Ӧ�ȣ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com