

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
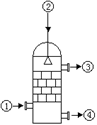
| A���Ӣٴ�ͨ�뺬SO3�Ļ�����壬�������ղ�����ȡ��������ʽ |
| B���Ӣڴ�����98.3%�����ᣬ�ɻ�������������Ӵ���� |
| C���Ӣ۴�������β���к�����ߵ�������SO2�����Բ���ֱ��������� |
| D���Ӣܴ��������ǿ���ˮ��ϡ����ϡ�͵ġ����̡����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ó���Ϊ0.05 mol��BaSO4 | B�����������SO2�����Ϊ0.448 L |
| C��a L�����������ʵ���Ϊ0.04 mol | D��a��ȡֵ��ΧΪ0.672<a<0.896 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
 ����������������ļ���ʽ���뻯��Ϊ_____________________________��
����������������ļ���ʽ���뻯��Ϊ_____________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NH4CuSO3����Ԫ�ر����� | B���̼�����ζ�������Ƕ������� |
| C���̼�����ζ�������ǰ��� | D���÷�Ӧ�������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A�� SO42�� SO42�� | B��S | C��SO32�� | D��S2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Al | B��Zn | C��Fe | D��Mg |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ʱ�� | ��ʼ | 8 h�� | 16 h �� | 24 h�� | 32 h�� | 4O h�� | 48 h�� |
| pH | 5��O | 4.8 | 4.5 | 4.3 | 4.2 | 4��O | 4��O |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com