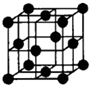
Dԭ������������������Ӳ�����3������DΪOԪ�أ�A�ĵ�������������壬��AΪHԪ�أ�A��Bԭ�ӵ�������֮�͵���Cԭ�Ӻ��ڵ���������D��Bԭ�Ӻ��������֮��ΪCԭ�Ӻ˵��������������B��������Ϊx����2��1+x��=8+x��x=6����BΪCԪ�أ���A��B��C��D��E���Ƕ���������Ԫ�أ���ԭ��������������E����������ԭ�Ӱ뾶����Ԫ�أ���EΪNaԪ�أ�
��1��EΪNaԪ�أ�ԭ������Ϊ11����Ԫ�����ڱ��е������ڵڢ�A�壬C��D��E���γɵļ����Ӻ�������Ų���ͬ����Ϊ10�����ӣ����ݺ˵����Խ�����Ӱ뾶ԽС��֪��
r��N
3-����r��O
2- ����r��Na
+�����ʴ�Ϊ���������ڵڢ�A�壻r��N
3-����r��O
2- ����r��Na
+����
��2��������ABC��һ�ּ������ᣬ�����к���4�����ۼ���ΪHCN������C��N֮�京��3�����õ��Ӷԣ�����ʽΪ
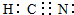
��D��E�γɵ����ӻ��������������Ӹ�����Ϊ1��2�����������ΪNa
2O
2��Na
2O����ΪNa
2O
2��O
2-�д��ڹ��ۼ�����ΪNa
2O�����ڹ��ۼ���
�ʴ�Ϊ��
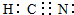
����һ�����ڹ��ۼ�����ΪNa
2O
2��O
2-�д��ڹ��ۼ�����ΪNa
2O�����ڹ��ۼ���
��3��A��B��D��E���γɶ��ֻ�������У��ס������ֻ��������Է�������֮��Ϊ42��41���������ֻ�����ֱ�ΪNaHCO
3��CH
3COONa����Է��������ֱ�Ϊ84��82������CH
3COOH���Խ�ǿ����NaHCO
3ˮ��̶ȴ���Һ����ǿ��ˮ������CH
3COOH����Һ�ʼ��ԣ�ˮ�ⷽ��ʽΪ CH
3COO
-+H
2O

CH
3COOH+OH
-��
�ʴ�Ϊ������ CH
3COO
-+H
2O

CH
3COOH+OH
-��
��4��250C��1OlkPa�£�BA4��ȼ����Ϊa kJ/mol��Ϊ�����ȼ�գ�
�Ȼ�ѧ����ʽΪCH
4��g��+2O
2��g���TCO
2��g��+2H
2O��l������H=-a kJ/mol���٣�
9gҺ̬ˮ��Ϊˮ��������b kJ����Ӧ���Ȼ�ѧ����ʽΪH
2O��l���TH
2O��g����H=+2bkJ/mol���ڣ�
���ø�˹���ɽ���+2���ڿɵ�CH
4��g��+2O
2��g���TCO
2��g��+2H
2O��g������H=-��a-4b�� kJ/mol��
�ʴ�Ϊ��CH
4��g��+2O
2��g���TCO
2��g��+2H
2O��g������H=-��a-4b�� kJ/mol��

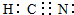




 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�


 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�