
| Ԫ�� | �����Ϣ |
| A | A�Ļ�̬ԭ�����������Ų�ʽΪ2S22P3 |
| B | B�ǵؿ��к�����ߵ�Ԫ�� |
| C | C+��B�ļ����ӵĵ��Ӳ�ṹ��ͬ |
| D | D��һ�ֺ��ص�������Ϊ64��������Ϊ35 |
| E��F | E��F��ͬ������ͬ�壬��ԭ������F��E��2 |

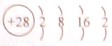 ��
��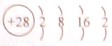 ��
��| 5+1-2��3 |
| 2 |
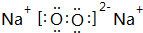 ��������FeCl2��Һ��Ӧ������Ӧ��Na2O2��FeCl2�����ʵ���֮��Ϊ1��2���������ƾ���ǿ�����ԣ���������������Ϊ�����ӣ����ݵ���ת���غ��֪����������ǡ�ñ���ȫ��������Ӧ����NaCl�����������غ��֪����FeCl3�����Ԫ���غ��֪����������������ƽ��÷�Ӧ�Ļ�ѧ����ʽΪ3Na2O2+6FeCl2+6H2O�T6NaCl+4Fe��OH��3��+2FeCl3��
��������FeCl2��Һ��Ӧ������Ӧ��Na2O2��FeCl2�����ʵ���֮��Ϊ1��2���������ƾ���ǿ�����ԣ���������������Ϊ�����ӣ����ݵ���ת���غ��֪����������ǡ�ñ���ȫ��������Ӧ����NaCl�����������غ��֪����FeCl3�����Ԫ���غ��֪����������������ƽ��÷�Ӧ�Ļ�ѧ����ʽΪ3Na2O2+6FeCl2+6H2O�T6NaCl+4Fe��OH��3��+2FeCl3�� ��3Na2O2+6FeCl2+6H2O�T6NaCl+4Fe��OH��3��+2FeCl3��
��3Na2O2+6FeCl2+6H2O�T6NaCl+4Fe��OH��3��+2FeCl3��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�긣��ʡ����һ�и�һ��ѧ��ѧ�ο��Ի�ѧ�Ծ����������� ���ͣ������
��12�֣�A��B��C��D��E��F��ΪԪ�����ڱ�ǰ20������Ԫ�أ���˵����������������A��Bͬ���ڣ�B��Dͬ���������ڣ�A��B��D����Ԫ�صĺ˵����֮��Ϊ30��C��Eͬ���ڣ����γ�1��1�͵����ӻ����F����������������1����ش�
��1��A��B��C��D��E��F����Ԫ�ص�Ԫ�ط��ŷֱ�Ϊ_______________________ __��
��2���û�ѧʽ��ʾA��D��E����������ˮ����������ǿ������˳��_______��
��3���û�ѧʽ��ʾD��E�⻯��Ļ�ԭ����ǿ������˳��_______��
��4����д��C��E����������Ӧˮ����֮�䷴Ӧ�Ļ�ѧ����ʽ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ���Ĵ�ʡüɽ�и�����һ������Կ��Ի�ѧ�Ծ��������棩 ���ͣ������
A~F��ΪԪ�����ڱ���ǰ������Ԫ�أ��������Ϣ���±���
Ԫ�� �� �� �� Ϣ
A A�Ļ�̬ԭ�����������Ų�ʽΪ2s22p3
B B�ǵؿ��к�����ߵ�Ԫ��
C C����B�ļ����ӵĵ��Ӳ�ṹ��ͬ
D D��һ�ֺ��ص�������Ϊ64��������Ϊ35
E ��F E ��F��ͬ������ͬ�壬��ԭ������F��E��2
��ش��������⣺
��1��D�ļ۵��ӵĵ����Ų�ʽ�� ��Fԭ�ӵ�ԭ�ӽṹʾ��ͼΪ ��
��2��A��B�ĵ�һ�����ܵĴ�С˳��Ϊ ��
��3��AB3�� ��Aԭ�ӵ��ӻ��������Ϊ_______����A2B��Ϊ�ȵ�����ķ��ӵķ���ʽΪ ����дһ�����ɣ���
��4��D����ľ�����ͼ��ʾΪ�����������ܶѻ� (�ھ��� �Ķ�������ľ�����һ��Dԭ��)����D�ľ�����Dԭ�ӵ���λ��Ϊ ��

��5����֪17gA�ļ��⻯�������������̬ˮʱ�ų�QkJ����������д��A�ļ��⻯����������Ȼ�ѧ��Ӧ����ʽ ��
��6��C2B2�ĵ���ʽΪ____��������E�Ķ��Ȼ�����Һ��Ӧ������Ӧ��C2B2��E�Ķ��Ȼ�������ʵ���֮��Ϊ1��2����÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�츣��ʡ��һ��ѧ��ѧ�ο��Ի�ѧ�Ծ��������棩 ���ͣ��ƶ���
��12�֣�A��B��C��D��E��F��ΪԪ�����ڱ�ǰ20������Ԫ�أ���˵����������������A��Bͬ���ڣ�B��Dͬ���������ڣ�A��B��D����Ԫ�صĺ˵����֮��Ϊ30��C��Eͬ���ڣ����γ�1��1�͵����ӻ����F����������������1����ش�
��1��A��B��C��D��E��F����Ԫ�ص�Ԫ�ط��ŷֱ�Ϊ_______________________ __��
��2���û�ѧʽ��ʾA��D��E����������ˮ����������ǿ������˳��_______��
��3���û�ѧʽ��ʾD��E�⻯��Ļ�ԭ����ǿ������˳��_______��
��4����д��C��E����������Ӧˮ����֮�䷴Ӧ�Ļ�ѧ����ʽ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com