
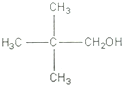
���� ������A�Ľṹ��ʽ��ͼ�� ������-CH2OH���ɷ�����������BΪC��CH3��3CHO��CΪC��CH3��3COOH��
������-CH2OH���ɷ�����������BΪC��CH3��3CHO��CΪC��CH3��3COOH��
��� �⣺������A�Ľṹ��ʽ��ͼ�� ������-CH2OH���ɷ�����������BΪC��CH3��3CHO��CΪC��CH3��3COOH��
������-CH2OH���ɷ�����������BΪC��CH3��3CHO��CΪC��CH3��3COOH��
��1���Ҵ������ǻ����������Ը�����ط���������ԭ��Ӧ�����Ậ���Ȼ����������ԣ��ɷ����кͷ�Ӧ������̼���Ʒ�Ӧ���ɶ�����̼���壬���ܼ����Ϊ�ڢܢݣ�
�ʴ�Ϊ���ڢܢݣ�
��2��AΪ ��CΪC��CH3��3COOH���ֱ����ǻ����Ȼ����������Ʒ�Ӧ���������������ʵ�����A��C�ֱ�������Na��Ӧ���ɵ�H2����ͬ״̬�µ������Ϊ1��1��
��CΪC��CH3��3COOH���ֱ����ǻ����Ȼ����������Ʒ�Ӧ���������������ʵ�����A��C�ֱ�������Na��Ӧ���ɵ�H2����ͬ״̬�µ������Ϊ1��1��
�ʴ�Ϊ��1��1��
��3��A��B�ķ�Ӧ����ʽΪ2C��CH3��3CH2OH+O2$\stackrel{������Cu��}{��}$2C��CH3��3CHO+2H2O���ʴ�Ϊ��2C��CH3��3CH2OH+O2$\stackrel{������Cu��}{��}$2C��CH3��3CHO+2H2O��
��4��A��B�ķ�Ӧ����ʽΪ2C��CH3��3CH2OH+O2$\stackrel{������Cu��}{��}$2C��CH3��3CHO+2H2O��B��C��Ӧ�ķ���ʽΪ2C��CH3��3CHO+O2$��_{��}^{����}$2C��CH3��3COOH��
��A��C�ķ���ʽΪΪC��CH3��3CH2OH+O2$\stackrel{������Cu��}{��}$C��CH3��3COOH+H2O��
�ʴ�Ϊ��C��CH3��3CH2OH+O2$\stackrel{������Cu��}{��}$C��CH3��3COOH+H2O��
���� ���⿼���л�����ƶϣ�Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬ע������л�������ŵ������Լ�ת����ϵ�����������Ϣ���ѶȲ���


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������ĵ缫��ӦΪH2O2+2H++2e-�T2H2O | |
| B�� | ����ܷ�ӦΪMg+H2O2�TMg��OH��2 | |
| C�� | ����ʱ��������Χ��ˮ��pH��С | |
| D�� | ��ع���ʱ����Һ�е�H+���ƶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| X | Y | �������Һ | |
| A | Zn | Cu | ZnCl2��Һ |
| B | Cu | Zn | ϡH2SO4 |
| C | Cu | Zn | CuSO4��Һ |
| D | Zn | Zn | CuSO4��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
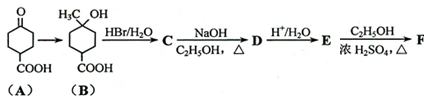
 ���ٺ˴Ź���������2�����շ� ���ܷ���������Ӧ
���ٺ˴Ź���������2�����շ� ���ܷ���������Ӧ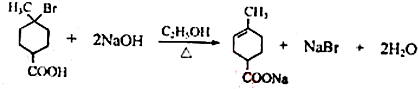 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ��������ķ���ʽΪC26H22Cl6O8 | |
| B�� | 1mol�����������������ϡ��Һ��Ӧʱ��������±�ز�ˮ�⣩���������6mol NaOH | |
| C�� | �����������������ˮ����Եõ������л��� | |
| D�� | 1mol���������������ȫ��Ӧ����Ҫ����10mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
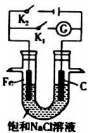
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
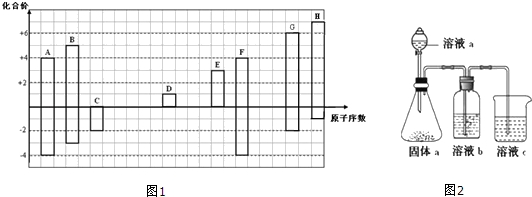
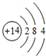 ��
��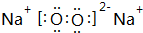 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ�����Ҵ����������Ȼ�̼ | |
| B�� | �����Ը��������Һ���𱽡���ϩ�ͼ��� | |
| C�� | ��̼������Һ�����Ҵ���������������� | |
| D�� | ��ȼ�շ�������顢��ϩ����Ȳ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | OH- | B�� | Fe | C�� | H+ | D�� | SCN- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com