
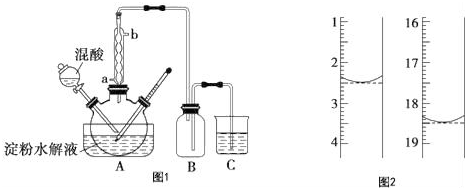
���� ��1������Ũ������������Խ�Ϸ�Ӧ���ʵ��ټ���98%����������Ŀ���ǣ��ӿ����ˮ������ʣ�������������ã���
��2����ˮ�������ۻ�����������Һ�����ۣ������ˮû�б仯��
��3�������ܵ������������������������������ã���������ˮ������Ϊ������
��4���л�������װ���ܷ�ֹ������
��5��NO�е�Ԫ��Ϊ+2�ۣ�NO2�е�Ԫ��Ϊ+4�ۣ��ڼ��������£�������̬���з�Ӧ���ŵ㣺���HNO3�����ʣ���ѭ��ʹ�õ�������� ȱ�㣺NOx������������ղ���ȫ��
��6���������Ϊ�Ϻ�ɫ�������в���Ҫ��ָʾ���������������£�������������ܺͲ��ᷢ��������ԭ��Ӧ���ɶ��������ӡ�������̼��ˮ�����ݷ�Ӧ���㣮
��� �⣺��1��Ũ�������ǿ�����ԡ���ˮ�Ժ���ˮ�ԣ�����ʵ���ǽ�C6H12O6���������������Ʊ����ᣬŨ������������Ũ������ˮ�����������ɲ���ķ����ƶ�������ʵ��ټ���98%����������Ŀ���ǣ��ӿ����ˮ������ʣ�������������ã���
�ʴ�Ϊ���ӿ����ˮ������ʣ�������������ã���
��2�������������ɫ�����Ѿ�ˮ��ĵ�����Һ�еμӼ��ε�Һ����Һ����ɫ����֤������û����ȫˮ�⣻��Һ������ɫ����֤��������ȫˮ�⣬
�ʴ�Ϊ����ˮ��
��3�����ܵ������������������������������ã�����Ч������Ч���ã�����ˮ�Ľ�����a��b����
�ʴ�Ϊ��a��
��4��װ��B�������Ƿ�ֹ����װ�ú�����װ�ü䷢����������ȫƿ�����ã�
�ʴ�Ϊ������ȫƿ��
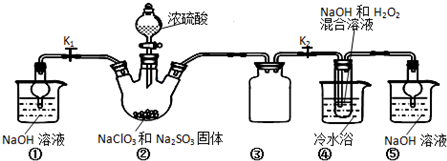
��5��������ӦΪ���з�Ӧ������NԪ�صĻ��ϼۿ�֪Ӧ����NaNO2����Ӧ�ķ���ʽΪNO+NO2+2NaOH=2NaNO2+H2O��
�ʴ�Ϊ��NO2+NO+2NaOH=2NaNO2+H2O��
��6�����������ҺΪ�Ϻ�ɫ�����ﵽ�ζ��յ�ʱ���ٵ�����������Һʱ������ɫ������ȥ�������ƣ�Na2C2O4������ϡ�����У�Ȼ�������Ը��������Һ���еζ������ӷ���ʽΪ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��n��KMnO4��=0.016L��0.0200mol•L-1=3.2��10-3mol�����ݷ���ʽ�ɵã�
2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��
2 5
3.2��10-3mol 8��10-3mol
��Ʒ�ж�ˮ�ϲ��������Ϊm=8��10-3mol��126g/mol=8��126��10-3g=1.008g��
��ò��ᾧ����Ʒ�ж�ˮ�ϲ������������Ϊ$\frac{1.008g}{1.2g}$��100%=84%��
�ʴ�Ϊ����ɫ������ɫ��84%��
���� ������Ҫ�����˲������ȡʵ�飬ע�����ʵ���ԭ������������������ԭ�����ǽ��Ĺؼ���Ҫ��߱�һ�������۷��������ͼ������������������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
 ԭ���������������A��B��C��D��E��F����Ԫ�أ�����A�Ļ�̬ԭ����3����ͬ�ܼ������ܼ��еĵ�������ȣ�C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ�ӵ���ͬ��DΪ������������ԭ�Ӱ뾶��������Ԫ�أ�E��F��Cλ��ͬһ���壬F���ڵ�һ�������ڣ�
ԭ���������������A��B��C��D��E��F����Ԫ�أ�����A�Ļ�̬ԭ����3����ͬ�ܼ������ܼ��еĵ�������ȣ�C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ�ӵ���ͬ��DΪ������������ԭ�Ӱ뾶��������Ԫ�أ�E��F��Cλ��ͬһ���壬F���ڵ�һ�������ڣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
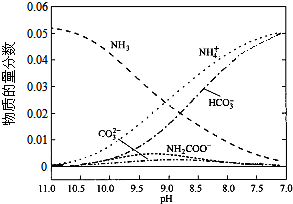
| A�� | ��pH=9.0ʱ��c��NH4+����c��HCO3-����c��NH2COO������c��CO32-�� | |
| B�� | ����CO2��ͨ�룬$\frac{c��O{H}^{-}��}{c��N{H}_{3}•{H}_{2}O��}$�������� | |
| C�� | ��ͬpH����Һ�д��ڹ�ϵ��c��NH4+��+c��H+���T2c��CO32-��+c��HCO3-��+c��NH2COO-��+c��OH-�� | |
| D�� | ����Һ��pH���Ͻ��͵Ĺ����У��к�NH2COO�����м�������� |
�鿴�𰸺ͽ���>>
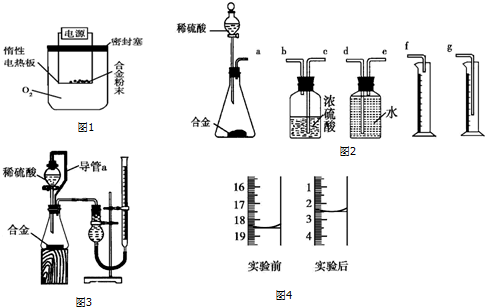
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

| ʵ����� | Ԥ����������� | ������ӷ���ʽ |
| ȡ����ʵ����еij�����Һ�������Լ�������KSCN��Һ | �����Һ�Ժ�ɫ�����ɫ����ΪFe3O4����֮��ΪFeO | Fe3++3SCN-�TFe��SCN��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | Na2SO4��Һ | B�� | FeCl3��Һ | C�� | Cu��NO3��2��Һ | D�� | ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��NaOH������ƽ��������е�ֽƬ�� | |
| B�� | ��NaOH�����ձ��У��ձ������ѳ��أ�����������ƽ��������� | |
| C�� | ������ȡ���궨Ϊ10.1 g�����������ƽ�ұߵ������� | |
| D�� | ������ȡ���궨Ϊ10 g�����������ƽ��ߵ������ϣ��������������Ƶ�0.1 gλ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com