
������ʵ���������ÿ�����þ��Ϊԭ����ȡ��������þ(Mg3N2)����֪ʵ���п��ܻᷢ�����з�Ӧ��
��2Mg+O2 2MgO����3Mg+N2
2MgO����3Mg+N2 Mg3N2����2Mg+CO2
Mg3N2����2Mg+CO2 2MgO+C��
2MgO+C��
��Mg+H2O MgO+H2���� ��Mg3N2 +6H2O
MgO+H2���� ��Mg3N2 +6H2O 3Mg(OH)2+2NH3��
3Mg(OH)2+2NH3��
�ɹ�ѡ���װ�ú�ҩƷ����ͼ��ʾ(þ�ۡ���ԭ���۾��Ѹ��װ�����������ķ�Ӧ����ȫ�ģ�����װ�õ�ĩ������������)��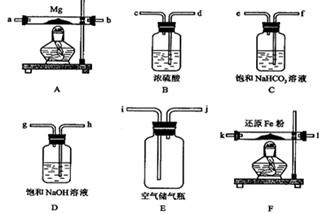
�ش��������⣺
��1�������ʵ�鷽��ʱ����װ��A��D��E�⣬��Ӧѡ���װ���� ������ĸ���ţ���ѡ��װ��DĿ��Ϊ_____________________________ ��
��2��ͨ����Ӧ�ȵ�ȼ ���ľƾ��ƣ����ͬʱ��ȼA��Fװ�õľƾ��ƣ�����ʹʵ���� ���ƫ�ߡ���ƫ�͡���ԭ��
��3�������һ��ʵ�飬��֤������Mg3N2��д���������衢����ͽ��ۣ�
_____________________________________________________________________
��1��BF ��2�֣� D Ŀ���dz�ȥ�����е�CO2�����ⷴӦ�۷�������2�֣�
��2��F����1�֣�ƫ�ͣ���2�֣����װ��F�еĻ�ԭ����û�дﵽ��Ӧ�¶�ʱ���������ܳ�����������ͬþ��Ӧ����ʹ����þ�л�������þ����2�֣�
��3��ȡ������������Թ��У��μ�����ˮ������ʪ�ĺ�ɫʯ����ֽ�����Թܿڣ�����Թ��е���Һ���ֻ��ǣ���ɫʯ����ֽ�����������֤���е���þ���ɡ���2�֣�
���������������1���÷�Ӧ��Ϊ�˵õ������ĵ���þ�������Ӱ��IJ��������ˮ����������ȥ����װ��B��������ȥˮ�����ģ�����A֮ǰ��F����������ȥ������D Ŀ���dz�ȥ�����е�CO2�����ⷴӦ�۷�����
��2����һװ���У��ܶ��˿��ܻῼ�ǵ�Fװ����������ȥˮ�����ģ������Ļ�����Ӧ������������ô�죬������װ����������ȥ�����ġ����װ��F�еĻ�ԭ����û�дﵽ��Ӧ�¶�ʱ���������ܳ�����������ͬþ��Ӧ����ʹ����þ�л�������þ����2�֣�
��3����֤������Mg3N2���������õ���þ��������Ӧ��ȡ������������Թ��У��μ�����ˮ������ʪ�ĺ�ɫʯ����ֽ�����Թܿڣ�����Թ��е���Һ���ֻ��ǣ���ɫʯ����ֽ�����������֤���е���þ���ɡ�
���㣺����þ��ȡ���й�֪ʶ



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(19��)��ͭ�����Ҫ�ɷ���CuFeS2(��Ԫ����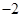 �ۣ���Ԫ����+2��)��ʵ�������û�ͭ��Ϊԭ����ȡ����ͭ������(Fe2O3)���������£�
�ۣ���Ԫ����+2��)��ʵ�������û�ͭ��Ϊԭ����ȡ����ͭ������(Fe2O3)���������£�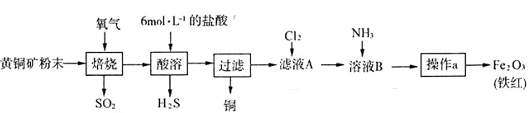
��֪��
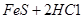 =
=
��1����ʵ�����У�Ӧ����ͭ���ĩ����__________(����������)�б��ա�
��2������Ӧ�����в�����SO2��H2Sͨ����ͼ��ʾװ���м������ǵ����ʡ���ʵ��֤��SO2����_________�Ժ�__________�ԡ�
��3����ѡ�����в���װ����ʵ��������MnO2��Ũ����Ϊԭ����ȡ����������������� 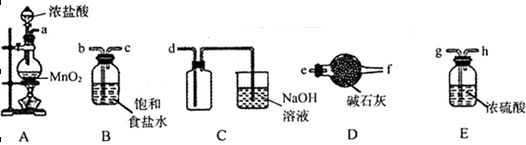
��Բ����ƿ�з�����Ӧ�����ӷ���ʽΪ_________________________________________��
�ڰ��������������ң����ܿ�����˳��Ϊa��__________________________________��
��װ�����Ӻú���װҩƷǰ�������װ�õ������ԣ����巽����__________________��
������ҺA��ͨ��C12��ijͬѧȡͨ��C12�����Һ�μ�KSCN��Һ������֤��C12�������Ա�Fe3+ǿ�Ľ��ۡ���ʵ������Ƿ����________(��ǡ���)�����ü�Ҫ���ֽ����������_____________________________________________________________��
��4����ʹ��20 g��ͭ���ĩ���Ƶ�8 gFe2O3(����)����û�ͭ���к�CuFeS2������������________ (���������Ӧ����ȫ�����ҹ����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ͭ�����׳ơ������������������������������й㷺��Ӧ�á�ij�����о�С���ͬѧ�ô�ͭ�ۣ�����̼�����ʣ�����������Ʊ�������;�������ⶨ�����нᾧˮ�ĺ�������Ƶ��������£�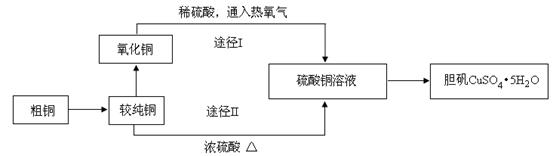
��1�����ϴ�ͭ��ת��Ϊ����ͭʱ��Ӧ�������� �ڽ������գ���д�������ƣ�������ͭ���������֬�������ȼ���Һϴȥ��ԭ���� �������ա���ͭ������õIJ����ǻ�������ͭ������ͭ����������ͭ�Ŀ���ԭ���� ��
a�����չ����в�������ͭ����ԭ b����������ͭ������������
c������ͭ�ڼ��ȹ����зֽ�����ͭ d�����ղ����ͭδ����ȫ����
��2��ͨ��;��Iʵ���ô�������ͭ��ȡ������������е�ʵ����������ǣ����ܡ�����ͨ���������ˡ� ����ȴ�ᾧ�� ����Ȼ����Ƚ��ɴ�������ͭ��ȡ����������;����;���������Ե������ŵ㣺
���������������������� ���� ��
�� ��
��3���ⶨ����������ᾧˮ�ĺ���ʱ�����ⶨ������������㣬���������ԭ�������___________��
a�����Ⱥ�����δ�������������ȴ
b��������μ��Ⱥ���������ϴ�
c������ǰ����ʱ����δ��ȫ����
d�����ȹ�������������ʧ
��4��������ͼװ�ü�����ˮ����ͭ��ĩֱ����ȫ�ֽ⣬A���Թ���ʣ���ɫ��ĩ���ô����ǵ�ľ�����뼯��ƿD������ľ���ܸ�ȼ����Ӧǰ���װ�õ�������ͼ�·��ı�����ʾ��
��ͨ�����㣬�ƶϸ�ʵ������������ͭ�ֽ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧ����������װ��̽����Ȫʵ�顣����A��F�ֱ�Ϊ����������ķ���װ�ã�CΪ������������������ⷴӦ��װ�á�
��ش��������⣺
��1��װ��F�з�����Ӧ�Ļ�ѧ����ʽ ��
��2��װ��A�еķ�Һ©����Һ��a��ѡ�� ��ѡ������ѡ��Ĵ��ţ�
A������ B��Ũ���� C��ϡ���� D��ϡ����
��3�����߿���Ӧ���ӱ�Ҫ�ij���װ�ã������ͼ�ġ���ѡװ�á���ѡ�����װ�õı�ţ��������пո�B__________��D__________��E__________��
��4����K1��K2��������ѹ�µ�H2S��Cl2�������1:1������ƿ����ƿ�з����ķ�Ӧ�û�ѧ����ʽ��ʾΪ ���ر�K1��K2��ʼ�տ�������ƿ�ڲ�������Ȫ�����������ǣ� ��
��5���ڲ�����4���Ļ����ϣ�������Ȫ���������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС��ͬѧչ����Ư����������(NaClO2)���о���
ʵ�����ȡNaClO2����
��֪��NaClO2������Һ���¶ȵ���38 ��ʱ�����ľ�����NaClO2��3H2O������38 ��ʱ�����ľ�����NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��������ͼ10��ʾװ�ý���ʵ�顣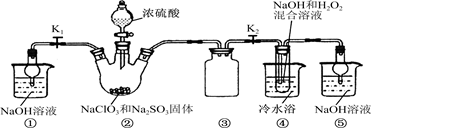
��1��װ�â۵�������____________________��
��װ�â��в���ClO2�Ļ�ѧ����ʽΪ____��
װ�â����Ʊ�NaClO2�Ļ�ѧ����ʽΪ____��
��3����װ�âܷ�Ӧ�����Һ���NaClO2����IJ�������Ϊ��
�ټ�ѹ��55 �������ᾧ���ڳ��ȹ��ˣ���____________���ܵ���60 �����õ���Ʒ��
ʵ��ⶨij����������Ʒ�Ĵ��ȡ�
�������ʵ�鷽����������ʵ�飺
��ȷ��ȡ��������������Ʒm g���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ(��֪��ClO2-��4I����4H��=2H2O��2I2��Cl��)�������û��Һ���250 mL������Һ��
����ȡ25.00 mL������Һ����ƿ�У��Ӽ��ε�����Һ����c mol��L��1 Na2S2O3��Һ�ζ������ζ��յ㡣�ظ�2�Σ����ƽ��ֵΪV mL(��֪��I2��2S2O32-=2I����S4O62-)��
�ȴﵽ�ζ��յ�ʱ������Ϊ________________��
�ɸ���Ʒ��NaClO2����������Ϊ____________(�ú�m��c��V�Ĵ���ʽ��ʾ)��
���ڵζ�������ȷ���������£���ʵ���ý��ƫ�ߣ�ԭ�������ӷ���ʽ��ʾΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ�����Կ�����O2�������20%)Ϊԭ�ϣ�����̼����ˮ�Ļ����£�����ͼAװ���Ʊ�������3O2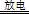 2O3����
2O3����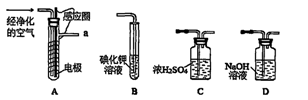
��1������ͨ��Aװ��֮ǰ��Ӧ�Ⱥ�ͨ������װ���е� �� ����װ����ţ���
��2��������⻯����Һ��ӦΪ��2KI+O3+H2O=2KOH+I2+O2����a������ͨ��װ��B����Һ�е�����Ϊ ��
��3��Ϊ�ⶨO2ת��ΪO3��ת���ʣ���װ��B�е���Һȫ��ת����һ�����У�����CC14������ȡ����Һ��������ȴ�����أ���I2����0.254g��
����ȡ�������ò������������� ��
����ʵ��ʱͨ�����1.12L����״������O2��ת����Ϊ ��
�۲ⶨʱ����A��Bװ�ü�����װ��D��ԭ���� ��
��4����ҵ�Ϸ���O3��O2���ɽ��������Һ�����ٷ��룬���з��뷽���������� ������ţ���
A�����ˡ�B������C����Һ��D����ȡ
��5�����������ں�CNһ���Ե�Ʒ�ˮ�Ĵ�������i����CNһת��ΪOCN������ii����OCNһ����ת��ΪCO32һ�����ֵ������塣����ii��ת��ʱ��O3��OCN�����ʵ�����֮��Ϊ3��2���ò���Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�⻯�ƹ����ǵ�ɽ�˶�Ա���õ���Դ�ṩ����ij��ȤС����ѡ������װ���Ʊ��⻯�ơ�
�ش��������⣺
��1�����װ��E�����ԵIJ��������� ��
��2����������װ����ȡ�⻯��ʱ��������������˳��Ϊi��___��___��___�� �� �� ��a���������ӿڵ���ĸ��ţ���
��3������������ʵ��װ�ý���ʵ�飬ʵ�鲽�����£����װ�������Ժ�װ��ҩƷ����Һ©��������__________________���밴��ȷ��˳���������в���ı�ţ���
| A�����ȷ�Ӧһ��ʱ�� | B���ռ����岢�����䴿�� |
| C���رշ�Һ©������ | D��ֹͣ���ȣ������ȴ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ʽ̼��ͭ��һ����;�㷺�Ļ���ԭ�ϡ���ҵ�Ͽ������Կ�ʴ��Һ����Ҫ����Cu2+��Fe2+��Fe3+��H +��Cl-���Ʊ������Ʊ��������£�
Cu2+��Fe2+��Fe3+���ɳ�����pH���£�
| �� �� | Cu(OH)2 | Fe (OH)2 | Fe (OH)3 |
| ��ʼ����pH | 4.2 | 5.8 | 1.2 |
| ��ȫ����pH | 6.7 | 8.3 | 3.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ͼװ�òⶨˮ���⡢��Ԫ�ص������ȣ��䷽���Ƿֱ�ⶨͨ����ǰ�����ܵ��������U�ιܵ������ʵ����m(H)��m(O)>1��8�����жԵ�����һ�����ԭ��ķ����У�һ���������(����)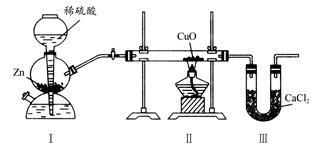
| A����װ��֮��ȱ�ٸ���װ�� |
| B����װ�ú�ȱ�ٸ���װ�� |
| C����װ���в���������ˮ���� |
| D��CuOû��ȫ������ԭ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com